
Neural Control of Eye Movements
Clifton M. Schor
INTRODUCTION
Three Fundamental Visual Sensory-Motor Tasks
Three Components of Eye Rotation
Binocular Constraints on Eye Position Control
Feedback and Feedforward control systems
Hierarchy of Oculomotor Control
Final Common Pathway
1. Cranial Nerves: III, IV, & VI and Motor Nuclei
2. Motor Neuron Response
FUNCTIONAL CLASSIFICATION INTO THREE GENERAL CATEGORIES
I. Stabilization of Gaze Relative To The External World
A. Extra-Retinal Signals (VOR)
B. Retinal Signals (OKN)
C. Neuro-Control of Stabilization Reflexes
1. Vestibulo-Ocular Reflex
2. Optokinetic Nystagmus
II. Foveal Gaze Lock (Maintenance of Foveal Alignment with Stationary and Slowly Moving Targets)
A. Static Control of Eye Alignment (Fixation):
B. Dynamic Control of Eye Alignment (Smooth Tracking Responses to Open and Closed-Loop Stimuli):
1. Conjugate Smooth Pursuit Tracking
2. Disconjugate Smooth Vergence Tracking
3. Adaptable Interactions between Smooth Pursuit and Smooth Vergence
C. Neuro-Control of Smooth Foveal Tracking
1 Smooth Pursuit Tracking System
2. Smooth Vergence Tracking System.
III. Foveal Gaze Shifts: Target Selection and Foveal Acquisition.
B Disconjugate Shifts of Gaze Distance (The Near Response in Symmetrical Convergence)
C. Interactions between Conjugate and Disconjugate Eye Movements (Asymmetric Vergence).
1. Saccadic Gaze Shifting System
2. Vergence Gaze Shifting System
NEUROLOGICAL DISORDERS OF THE OCULOMOTOR SYSTEM
II Gaze Restriction
III. Saccade Disorders
INTRODUCTION
Three Fundamental Visual Sensory-Motor Tasks
The neural control of eye movements is organized to optimize performance of three general perceptual tasks. One task is to resolve the visual field while we move either by translation or rotation through space (self motion). Our body motion causes the image of the visual field to flow across the retina and reflexive eye movements reduce or stabilize this image motion to improve visual performance. The second task is to resolve objects whose position or motion is independent of the background field (object motion). Eye movements improve visual resolution of individual objects by maintaining alignment of the two foveas with both stationary and moving targets over a broad range of directions and distances of gaze. The third task is to explore space and shift attention from one target location to another. Rapid eye movements place corresponding images on the two foveas as we shift gaze between targets lying in different directions and distances of gaze.
Three Components of Eye Rotation
All three perceptual tasks require three-dimensional control of eye position. These dimensions are controlled by separate neural systems. As described in the chapter on Kinematics, three pairs of extraocular muscles provide control of horizontal, vertical and torsional position of each eye. Eye movements are described as rotations about three principal axes as illustrated in Figure 1. Horizontal rotation occurs about the vertical Z axis, vertical rotation about the horizontal X axis and torsion about the line of sight or Y axis. As described in the chapter on Kinematics, the amount of rotation about each of the three principal axes that is needed to describe a certain direction of gaze and torsional orientation of the eye depends upon the order of sequential rotations (e.g. horizontal, followed by vertical and then torsional) (Tweed, 1997). Some oculomotor tasks, such as retinal image stabilization, utilize all three degrees of freedom whereas other tasks, such as voluntary gaze shifts, only require two degrees of freedom, i.e. gaze direction and eccentricity from primary position. As described by Donder’s law, torsional orientation of the eye is determined by horizontal and vertical components of eye position. Ocular torsion is independent of the path taken by the eye to reach a given eye position and is constrained by the gaze direction. Listing’s law quantifies the amount of ocular torsion at any given eye position, relative to the torsion of the eye in primary position of gaze.

Fig. 1 The three principal axes of eye rotation. Horizontal rotation occurs about the vertical axis (Z), vertical rotation about the transverse axis (X), and torsion about the anterior-posterior axis (Y).
Binocular Constraints on Eye Position Control
Binocular alignment of retinal images with corresponding retinal points places additional constraints on the oculomotor system. Because the two eyes view the world from slightly different vantage points, the retinal image locations of points subtended by near objects differ slightly in the two eyes. This disparity can be described with three degrees of freedom (horizontal, vertical and torsional components) that are analogous to the angular rotations of the eye shown in Figure 1. The main task of binocular eye alignment is to minimize horizontal, vertical and cyclodisparities subtended by near targets on the two foveas. This requires a conjugate system that rotates the two eyes in the same direction and amount, and a disconjugate system that rotates the visual axes in opposite directions. As described by Hering (1868), a common gaze direction for the two eyes is achieved by a combination of conjugate and disconjugate movements that are controlled by separate systems. The version system controls conjugate movements and the vergence system controls disconjugate movements. Pure version and vergence movements are described respectively by the isovergence and isoversion contours shown in Figure 2. The isovergence circle describes the locus of points that stimulate the same vergence angle in all directions of gaze (see Ono, 1983 for a review). A different isovergence circle exists at each viewing distance. The isoversion lines describe the locus of points that stimulate the same version angle over a range of viewing distances in a common direction of gaze relative to the head. Pure vergence movements occur along any of the isoversion lines and not just along the central or midsagittal plane. Fixation changes along any other contour result from a combination of version and vergence movements. Both version and vergence movements are described as combinations of horizontal, vertical and torsional rotations. For example there can be horizontal and vertical version and vergence movements. Torsional rotations are usually referred to as cyclo rotations (e.g. cycloversion or cyclovergence). Hering’s law implies that there is equal innervation of yoked muscle pairs. "… one and the same impulse of will directs both eyes simultaneously as one can direct a pair of horses with single reins." The law should not be taken literally, because common gaze commands from higher levels are eventually parceled into separate innervation sources in the brainstem that control individual muscles in the two eyes.
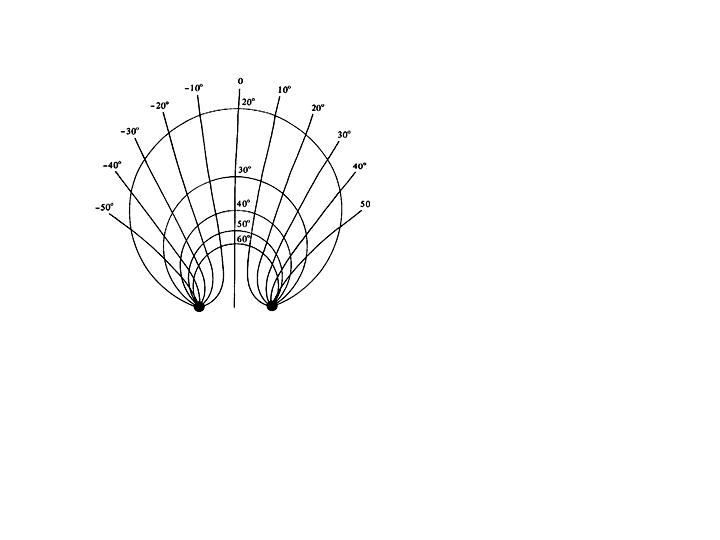
Fig. 2 Geometric representation of the two components of eye movements by locations of the intersections of the two visual axes. The value in degrees marked along each isovergence circle denotes the convergence angle and the value in degrees marked on the hyperbola isoversion lines denotes the visual direction when a line is not very close to the eyes.
Feedback and Feedforward control systems
The oculomotor system requires feedback to optimize sensory stimuli for vision with a sufficiently high degree of precision.. Feedback provides information about motor response errors based upon their sensory consequences, such as unwanted retinal image motion or displacement. This visual error information usually arrives too late to affect the current movement, because the time delays in the visual system are about 50-100 msec. Instead, it is used to adaptively adjust motor responses to minimize subsequent errors. Oculomotor systems use sensory information to guide eye movements in two different ways. Motor responses can be guided in a closed-loop mode with an ongoing feedback signal that indicates the difference between the desired and actual motor response, or they can operate without concurrent feedback in an open-loop mode. The closed-loop feedback mode is used to reduce internal system errors or external perturbations. A physical example of a closed-loop system is the thermostatic regulation of room temperature, e.g., if the outside temperature drops, the furnace will turn on so that the room temperature stays constant. Motor responses can also be controlled in an open-loop mode, without a concurrent feedback signal. A physical example of an open-loop system is a water faucet, e.g., if the pressure drops, the flow of water will also drop, because the valve does not compensate for the pressure drop.
The mode of the response depends on the latency of the response, its duration and velocity. In most examples, visual feedback in a closed-loop system is used to maintain or regulate a fixed position or slow movements of the eyes when there is adequate time to process the error signal. Errors in eye posture or movement are sensed from displacement of the object of regard from the fovea or slippage of the retinal image and negative feedback control mechanisms attempt to reduce the errors to zero during the response.
Feed-forward control systems do not utilize concurrent visual feedback and are described as open-loop. These systems can respond to non-visual (extra-retinal) stimuli, or they respond to advanced visual information with short latencies and brief durations. For example, brief rapid head movements stimulate vestibular signals that evoke compensatory eye movements to stabilize the retinal image. These head movements can produce retinal image velocities of 300-400 deg/sec, yet the eyes respond with a counter rotation within 14 msec of the movement (Lisberger, 1984). The oculomotor response to head motion must rely on vestibular signals since retinal image velocities produced by head rotation exceed the upper velocity limit for sensing motion by the human eye. Retinal image velocities that exceed this upper limit appear as blurred streaks rather than as moving images. Visual feedback is not available when the response to head rotation begins because the latency is too short to utilize concurrent visual feedback. A minimum of 50 msec. is needed to activate cortical areas that initiate ocular following (Miles, Kawano and Optican, 1986) such that any motor response with a shorter latency must occur without concurrent visual feedback. Some open-loop systems, such as brief rapid gaze shifts (saccades), respond to visual information sensed prior to the movement rather than during the movement. Their response is too brief to be guided by negative visual feedback. Accuracy of a feedforward system is evaluated after the response is completed. Visually sensed post-task errors are used by feedforward systems to improve the accuracy of subsequent open-loop responses in an adaptive process that calibrates motor responses. Calibration minimizes motor errors in systems that do not use visual feedback during their response. All feed-forward oculomotor systems are calibrated by adaptation and this plasticity persists throughout life.
Hierarchy of Oculomotor Control
The following sections present a functional classification of eye position and movement control systems used to facilitate three general perceptual tasks and a hierarchial description of their neuro-anatomical organization. A hierarchy of neural control exists within each of the functional categories of eye movements that plans, coordinates and executes motor activity. Three pairs of extraocular muscles that rotate each eye about its center of rotation are at the bottom of this hierarchy. The forces applied by these muscle pairs to the eye are controlled at the level above by the motor nuclei of the III, IV and VI cranial nerves. Motor neurons in these nuclei make up the final common pathway for all classes of eye movements. Axon projections from these neurons convey information to the extraocular muscles for executing both slow and fast eye movements. Above this level, premotor nuclei in the brainstem coordinate the combined actions of several muscles to execute horizontal, vertical, and torsional eye rotations. These gaze centers orchestrate the direction, amplitude, velocity, and duration of eye movements. Interneurons from the premotor nuclei all converge on motor nuclei in the final common pathway. Premotor neurons receive instructions from supranuclear regions including the superior colliculus, the substantia nigra, the cerebellum, frontal cortical regions including the frontal eye fields (FEF) and supplementary eye fields (SEF), and extrastriate regions including the medial temporal visual area (MT), the medial superior temporal visual area (MST), the lateral intraparietal area (LIP), and the posterior parietal area (PP). These higher centers plan the desired direction and distance of binocular gaze in 3-D space. They transform sensory visual stimuli into motor commands. They determine when and how fast to move the eyes to fixate selected targets in a natural complex scene or to return them to a remembered gaze location. The following sections will discuss the hierarchical control for each of three functional classes of eye movements. The next section describes the final common pathway that conveys innervation for all classes of eye movements.
Final Common Pathway
1. Cranial Nerves: III, IV, & VI and Motor Nuclei
Cranial nerves III, IV, and VI represent the final common pathway as defined by Sherrington (1947) for all classes of eye movements. All axon projections from these cranial nuclei carry information for voluntary and reflex fast and slow categories of eye movements (see Leigh and Zee, 1999 for a review). The oculomotor (III), trochlear (IV), and abducens (VI) nuclei innervate the six extraocular muscles, iris and ciliary body. The abducens nucleus innervates the ipsilateral lateral rectus. Premotor interneurons also project from VI to the contralateral oculomotor nucleus for control of the contralateral medial rectus, to produce yoked movements on lateral gaze that are consistent with Hering’s law. The trochlear nucleus innervates the contralateral superior oblique. The oculomotor nucleus innervates the ipsilateral medial rectus, inferior rectus, and inferior oblique, and the contralateral superior rectus. The anterior portion of the oculomotor nucleus also contains motor neurons that control pupil size and accommodation in a specialized region called the Edinger-Westphal nucleus (Gamlin Zhang Clendaniel and Mays 1994). Afferents from this nucleus synapse in the ciliary ganglion prior to innervating their target muscles (Westheimer and Blair 1973). The regions of the oculomotor nucleus that control various eye muscles are illustrated in Figure 3.
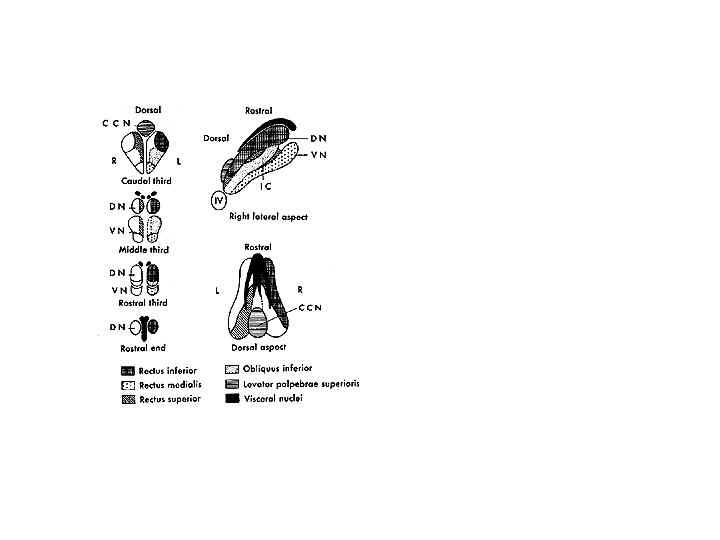
Fig. 3 . Representation of motor neurons for right extraocular muscles in the oculomotor nucleus of monkey. Transverse sections at levels as indicated in the complex. DN, dorsal nucleus; VN ventral nucleus, CCN caudal central nucleus; IC, intermediate column, IV trochlear nucleus. Lateral and dorsal views are shown to the right. (From Warwick R. J Comp Neural 1953) Adler’s figure 1992 page 137
2. Motor Neuron Response
The motor neurons control both the position and velocity of the eye. They receive inputs from burst and tonic cells in pre-motor nuclei. The tonic inputs are responsible for holding the eyes steady, and the more phasic or burst like inputs are responsible for initiating all eye movements to overcome orbital viscosity and for controlling eye movements. All motoneurons have the following characteristics as illustrated in Figure 4 (Robinson and Keller, 1972).
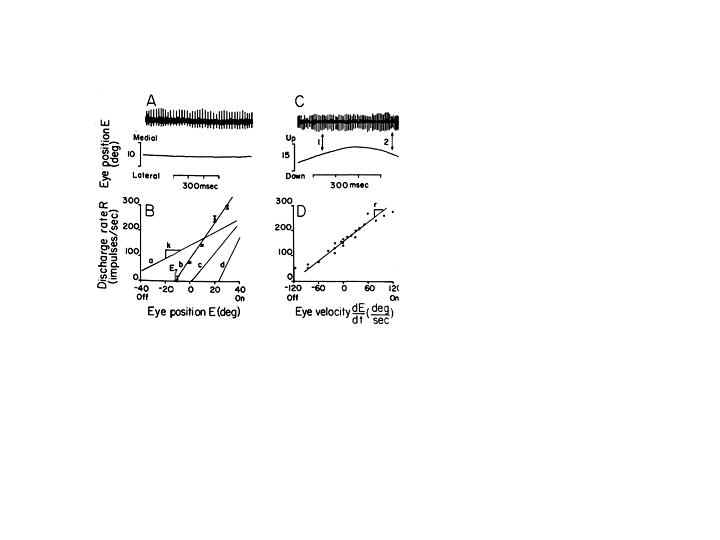
Fig. 4 Discharge rate of oculomotor neurons in relation to eye movement. On the left, the steady firing rate is shown when the eye is stationary; and below, the rate-position curve illustrates an increase of firing rate with eye position for four neurons. On the right, the firing rate is shown during a slow voluntary eye movement. The arrows indicate points where the eye passes through the same position with velocity of opposite signs, and the associated firing rate is different. Below, firing rate is plotted for a single unit and a particular deviation of the eye as a function of eye velocity. (from Robinson and Keller, 1972)
1. They have on-off directions (they increase their firing rate in the direction of agonist activity).
2. All cells participate in all classes of eye movements including steady fixation.
3. Each cell (especially tonic) has an eye position threshold at which it begins to fire. Motoneurons have thresholds that range from low to high. Cells with low thresholds begin firing when the eye is in the off field of the muscle that it innervates. Cells with higher thresholds can begin to fire after the eye has moved past the primary position by as much as 10 degrees into the on field of the muscle. The graded thresholds of motoneurons are responsible for the recruitment of active cells as the eyes move into the field of action for the muscle.
4. Increasing the frequency of spike potentials for a given neuron increases contractile force. Once their threshold is exceeded all cells increase their firing rate as the eye moves further along in the on direction of the muscle until they saturate. Cells increase their firing rate linearly as the eye moves into their on field.
FUNCTIONAL CLASSIFICATION INTO THREE GENERAL CATEGORIES
I. STABILIZATION OF GAZE RELATIVE TO THE EXTERNAL WORLD
Movements of the head during locomotion tasks such as walking are described by a combination of angular rotations and linear translations. The oculomotor system keeps gaze fixed in space during these head movements by using extra-retinal and retinal velocity information about head motion. The primary extra-retinal signal comes from accelerometers in the vestibular apparatus.
The vestibular system contains two types of organs that transduce angular and linear acceleration of the head into velocity signals (Figure 5) (see reviews by Simpson and Graf, 1995; Melvill Jones 1989). Three semicircular canals lie on each side of the head in three orthogonal planes that are approximately parallel to a mirror image set of planes on the contralateral side of the head. These canals are stimulated by brief angular rotations of the head and the resulting reflexive ocular rotation is referred to as the vestibulo-ocular reflex (angular VOR). In addition, two otoliths (the utricle and sacculus) transduce linear acceleration caused by head translation as well as head pitch (tilt about the interaural axis) and roll (tilt about the nasal-occipital axis) into translation velocity and head orientation signals (linear VOR). These signals stimulate eye rotations that are approximately equal and opposite to the motion of the head. This stabilization reflex has a short 7-15-msec latency because it is mediated by only three synapses (Lisberger 1984) and is accurate for head turns at velocities in excess of 300 deg/s (Keller 1978). Hair cells in the canals can be stimulated by irrigation of one ear with cold water. This produces a caloric-vestibular nystagmus that causes the eyes to rotate slowly to the side of the irrigated ear (see Cogan, 1956 for a review). These slow-phase movements are interrupted by fast saccadic eye movements that reset eye position in the reverse direction (fast phase). A sequence of slow and fast phases is referred to as jerk nystagmus (Figure 6). Head rotations about horizontal, vertical, and nasal-occipital axes produce VOR responses with horizontal, vertical and torsional counter-rotations of the slow phase of the nystagmus (Seidman and Leigh, 1989).
Fig. 5 Vestibular end-organs in the human temporal bone. Three canals transduce angular head acceleration and two otoliths, the sacculus and utricle, transduce linear acceleration and head orientation. Right labyrinth and chochlea are viewed from horizontal aspect. (Drawings by Ernest W. Beck: courtesy Beltone Electonics Corp., Chicago, Ill.) The canals are in three orthogonal planes that are approximately parallel to a mirror image set of planes on the contralateral side of the head that lie roughly in the pulling direction of the three muscle planes A, Lateral Canals. B, Anterior and posterior vertical canals. LC, lateral canal; AC anterior vertical canal: PC, posterior vertical canal. (From Barber HO, Stickwell CW: Manual of electronystagmography, St Louis, CV Mosby, 1976). Adler’ sfigures 1992 page 148 & 149)
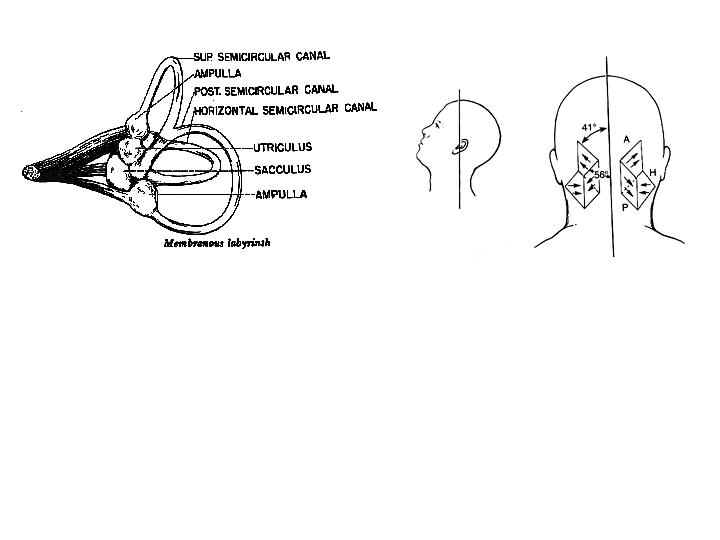
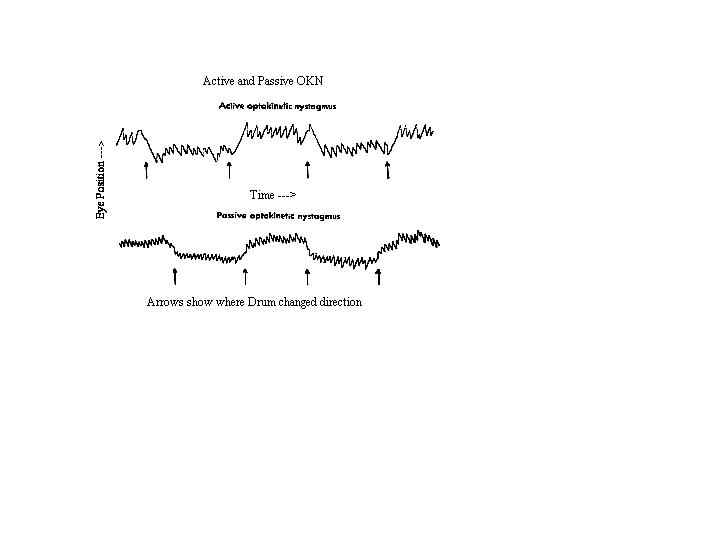
Fig. 6 OKN and VOR are composed of a slow phase (Beta) that rotates the eye in a direction that stabilizes the retinal image and a fast phase (alpha) that resets the eye’s position. The figure illustrates the vestibulo-ocular response to sustained rotation. The slow phase is in the direction opposite to head rotation. Horizontal position is plotted against time. The reflex gradually habituates and has disappeared by about 30 seconds. (Miller, 1982)
To be effective, these reflex eye rotations must stabilize the retinal image. If the axis of angular head rotation coincided with the center of eye rotation, perfect compensation would occur if angular eye velocity equaled angular head velocity. However, the axis of head rotation is the neck and not the center of eye rotation such that when the head rotates, the eyes both rotate and translate with respect to the visual field. This is exacerbated during near viewing conditions. To stabilize the retinal image motion of a nearby target caused by angular head movements, the eye must rotate more than the head. Indeed, the gain of the VOR increases with convergence (Snyder and King, 1992). Mismatches between eye and head velocity also occur with prescription spectacles that magnify or minify retinal image motion. Because the VOR responds directly to vestibular and not visual stimuli, its response is classified as open loop. The VOR compensates for visual errors by adapting its gain in response to retinal image slip to produce a stabilized retinal image (Melvill Jones, 1989). Perfect compensation would occur if angular eye velocity were equal and opposite to angular head velocity while viewing a distant scene. However empirical measures show that at high oscillation frequencies (2Hz) compensation by the VOR is far from perfect and yet the world appears stable and single during rapid head shaking without any perceptual instability or oscillopsis (Collewijn, Steinman, and Erkelens, 1991). Thus, to perceive a stable world, the visual system must be aware of both the amount of head rotation and the inaccuracy of compensatory eye movements so that it can anticipate any residual retinal image motion during the head rotation.
B. Retinal Signals:
Head motion also produces whole-field retinal image motion of the visual field (optic flow) (see Collewijn 1989 for a review). These retinal signals stimulate reflexive compensatory eye rotations that stabilize the retinal image during slow or long-lasting head movements. The eyes follow the moving field with a slow phase that is interrupted by resetting saccades (fast phase) 1 to 3 times per second (Cheng and Outerbridge, 1975). This jerk nystagmus is referred to as optokinetic nystagmus (OKN) and it complements the VOR by responding to low-velocity sustained head movements such as those that occur during walking and posture instability. Like the VOR, OKN also responds with horizontal, vertical and cyclo eye rotations to optic flow about vertical, horizontal and nasal-occipital axes (Cheung and Howard, 1991).
The optokinetic response to large fields has two components, including an early and delayed segment (OKNe and OKNd). OKNe is a short-latency ocular following response (< 50 msec.) that constitutes the rapid component of OKN (Miles Kawano and Optican, 1986), and OKNd builds up slowly after 7 seconds of stimulation (Raphan and Cohen, 1985). OKNe is likely to be mediated by the pursuit pathway (Miles, 1993). The delayed component is revealed by the continuation of OKNd in darkness (optokinetic after nystagmus, OKAN). OKNd results from a velocity memory or storage mechanism (see Collewijn 1985 and Raphan and Cohen, 1985 for reviews). The time constant of the development of the OKAN matches the time constant of decay of the cupula in the semicircular canals (Robinson, 1981). Thus OKAN builds up so that vision can compensate for loss of the vestibular inputs during prolonged angular rotation that might occur in a circular flight path. OKN can be used clinically to evaluate visual acuity objectively by measuring the smallest texture size and separation in a moving field that elicits the reflex.
C. Neuro-Control Of Stabilization Reflexes
1. Vestibulo-Ocular Reflex
The transducer that converts head rotation into a neural code for driving the VOR consists of a set of three semicircular canals paired on each side of the head (See Melvill Jones 1989 for a review). The horizontal canals are paired and the anterior canal on one side is paired with the posterior canal on the contralateral side. (Figure 5) These are opponent pairs so that when one canal is stimulated by a given head rotation its paired member on the contralateral side is inhibited. For example, downward and forward head motion to the left causes increased firing of the vestibular nerve for the left anterior canal and decreased firing of the vestibular nerve projections from the right posterior canal. The three canals lie roughly in the pulling directions of the three muscle planes (Melvill Jones 1989). Thus the left anterior canal and right posterior canal are parallel to the muscle planes of the left eye vertical recti and the right eye obliques. Pathways for the horizontal VOR are illustrated in Figure 7 for a leftward head rotation (Leigh and Zee, 1999). Excitatory innervation projects from the left medial vestibular nucleus to the right abducens nucleus to activate the right lateral rectus, and an interneuron from the right abducens nucleus projects to the left oculomotor nucleus to activate the left medial rectus. The abducens serves as a premotor nucleus to coordinate conjugate horizontal movements to the ipsilateral side in accordance with Hering’s law.
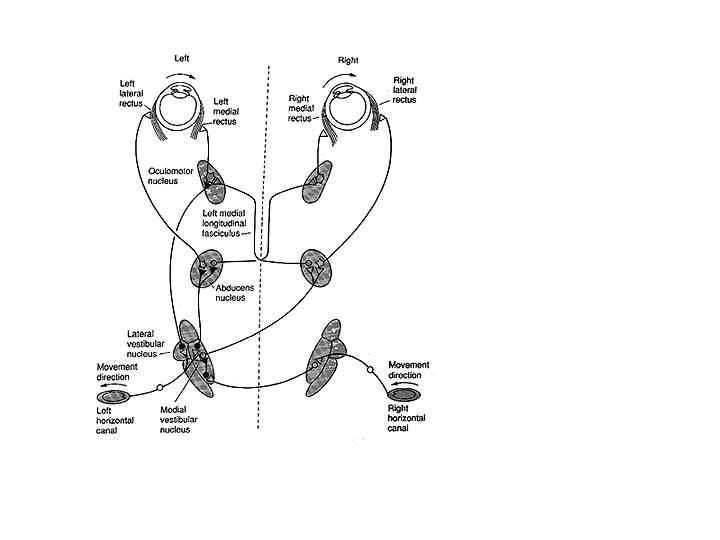
Fig. 7 Pathways of the horizontal VOR in the brain stem for leftward head rotation. Inhibitory connections are shown as filled neurons, excitatory connections as unfilled neurons. Leftward head rotation stimulates the left horizontal canal and inhibits the right horizontal canal. This results in an increased discharge rate in the right lateral and left medial rectus and decreased discharge rate in the left lateral and right medial rectus (From Goldberg, Eggers and Gouras, 1991).
The cerebellar flocculus is essential for adaptation of the VOR to optical distortions such as magnification. The flocculus receives excitatory inputs from retinal image motion (retinal slip) and head velocity information (canal signals) and inhibitory inputs from neural correlates of eye movements that provide a negative feedback signal (Lisberger, 1988). Adaptation only occurs if retinal image motion and head turns occur together (Ito, 1985). The gain of the VOR will be adapted to decrease whenever retinal slip and head turns are in the same direction, and to increase whenever they are in opposite directions. Following adaptation, an error correction signal is projected from the flocculus by Purkinje cells to floccular target neurons (FTN) in the vestibular nucleus to make appropriate changes in VOR gain (Lisberger, 1988).
2. Optokinetic Nystagmus
The visual stimulus for OKN is derived from optic flow of the retinal image (for reviews see Fuchs and Mustari, 1993; Wallman, 1993; Albright, 1993). The retina contains ganglion cells that respond exclusively to motion in certain directions or orientations. This information passes along the optic nerve, decussates at the chiasm and projects to the cortex via the geniculate body (LGN) or to the midbrain via the accessory optic tract (Hoffmann Kistler and Ilg, 1992) (Figure 8). This tract has several nuclei in the pretectal area. One pair of these nuclei, the nucleus of the optic tract (NOT), is tuned to horizontal target motion to the ipsilateral side (i.e. nasal to temporal motion). The lateral and medial terminal nuclei (LTN and MTN) are tuned for vertical target motion (Pasik and Pasik, 1964). Neurons in these nuclei only receive subcortical inputs from the contralateral eye. They have large receptive fields and respond to large textured stimuli moving in specific directions. Stimulation of the right NOT with rightward motion causes following movements of both eyes to the right or ipsilateral side, and similarly stimulation of the left NOT with leftward motion causes leftward conjugate following movements. Each NOT projects signals via the inferior olive to the vestibular nuclei and possibly to the flocculus via the climbing fibers of the cerebellum (Fuchs and Mustari, 1993). The NOT provides a visual signal to the vestibular nucleus, and the motor response is the same as for velocity signals originating from the semicircular canals.
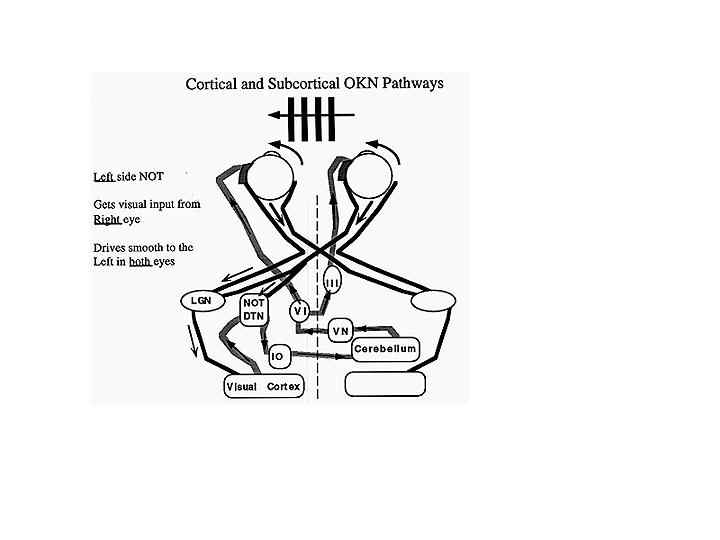
Fig. 8 Simplified schematic illustrating the inputs to the NOT from subcortical crossed retinal projections and cortical-tectal projections. Each NOT gets direct retinal input form the contralateral nasal retina, which is excited by temporal-to-nasal motion. It also receives indirect cortical input (CTX) from the temporal retina of the ipsilateral eye, which tends to be excited more by nasal-to-temporal motion as well as input via the accessory optic system (AAOS), and input from the contralateral visual cortex via the corpus callosum (adapted from Hoffman et al 1992)
The cortical region that organizes motion signals is the medial superior temporal lobe (MST). This region is important for generating motion signals for both pursuit and OKN (Albright, 1993). Binocular cortical cells receive projections from both eyes and code ipsilateral motion from the contralateral visual field at higher velocities than the subcortical system (Wallman, 1993). The cortical cells project to the ipsilateral NOT.
Until the age of 3-4 months, the monocular subcortical projections predominate because the cortical projection has not yet developed (see Schor 1993 for a review). As a result, OKN in infancy is driven mainly by the crossed subcortical input. The consequence is that monocular stimulation only evokes OKN with temporal-to-nasal motion but not with nasal-to-temporal motion. After 3-4 months of development, the infant’s cortical projections predominate and horizontal OKN responds to both monocular temporalward and nasalward image motion. The cortical projections to the NOT fail to develop in infantile esotropia and as adults these patients exhibit the same asymmetric OKN pattern as observed in immature infants (Schor, Wilson, Fusaro, and McKee 1997). This anomalous projection is responsible for a disorder known as latent nystagmus in which a jerk nystagmus occurs when one eye is occluded with the slow phase directed toward the side of the covered eye. During monocular fixation, the stimulated retina increases the activity of neurons in the contralateral NOT via subcortical crossed projections, but it is unable to innervate the ipsilateral NOT via the ineffective cortical-tectal projection. The result is that both eye’s positions are drawn to the side of the stimulated NOT (i.e. the side of the covered eye). The fixation error is corrected with a saccade and a repeated sequence is described as latent or occlusion nystagmus (Dell’Osso, Schmidt and Daroff 1979).
II. FOVEAL GAZE LOCK (MAINTENANCE OF FOVEAL ALIGNMENT WITH STATIONARY AND SLOWLY MOVING TARGETS)
A. Static Control of Eye Alignment (Fixation):
The oculomotor system enhances visual resolution by maintaining alignment of the fovea with attended stationary and moving targets (Westheimer and McKee, 1975) (See Kowler 1989 for a review). During fixation of stationary targets, the eyes sustain foveal alignment over a wide range of target locations in the visual field. Gaze direction is controlled by a combination of eye position in the orbit and head position (Land 1992). Gaze is mainly controlled by eye position for targets lying at eccentricities of less than 15 degrees from primary position (Bahill, Adler and Stark, 1975). Steady fixation at larger gaze eccentricities is accomplished with a combination of head and eye position. Holding eye fixation at large eccentricities (>30 degrees) without head movements is difficult to sustain and the eye drifts intermittently toward primary position in gaze evoked nystagmus (Able, Parker, Daroff and Dell’Osso 1977). This drift is exacerbated by alcohol (Baloh, Sharma, Moskowitz and Griffith, 1979). Even within the 15-degree range, the fixating eye is not completely stationary. It exhibits physiological nystagmus that is composed of slow horizontal, vertical and torsional drifts (0.1 deg/sec), micro-saccades (< 0.25 deg), and a small amplitude (< 0.01 deg) high frequency tremor (40-80Hz) (Carpenter, 1988). Some of the drifts and saccades are error producing, while others are error correcting when they serve to continually minimize motion and adjust the alignment of the target of regard with the fovea (Kowler, 1989; Van Rijn Van der Steen and Collewijn, 1994). The high frequency tremor reflects the noise possibly originating from asynchronous firing of individual motoneurons that is filtered by the mechanical properties of the eye.
Stereoscopic depth perception is enhanced by accurate binocular fixation (see Schor 1983 and 2000 for reviews). Precise bifoveal alignment requires that the eyes maintain accurate convergence at the distance of the attended target. Small constant errors of convergence during attempted binocular fixation (< 15 arc min) are referred to as fixation disparity (see Ogle Martens, Dyer 1967 for a review) and these can impair stereo performance (Badcock and Schor 1985). Fixation disparity is a closed-loop error because it occurs in the presence of retinal image feedback from binocular disparity. Fixation disparity results from incomplete nullification of an open-loop error of convergence known as heterophoria. Heterophoria equals the difference between the convergence stimulus and the convergence response measured under open-loop conditions (e.g. monocular occlusion). The magnitude of fixation disparity increases monotonically with the disparity stimulus to convergence when it is varied with horizontal prisms (Ogle et al 1967). The slope of this function is referred to as the forced-duction fixation disparity curve (Ogle et al, 1967). Shallow slopes of these curves indicate that the heterophoria is reduced by adaptation during binocular or closed-loop conditions (Schor, 1983). Vergence adaptation is very rapid. For example, when convergence is stimulated for only one minute with a convergent disparity and then one eye is occluded, the convergence response persists in the open-loop state (Schor 1983). Typically the convergent disparity is produced by prisms that deflect the perceived direction of both eye’s images inward, or in the nasalward direction, (i.e. the base of the prism before each eye is temporalward or "base-out"). This adapted change in heterophoria is constant in all directions of gaze and is referred to as concomitant adaptation. Prism adaptation is in response to efforts by closed-loop disparity vergence to nullify the open-loop vergence error (heterophoria). Prism adaptation improves the accuracy of binocular alignment and stereo-depth performance.
Horizontal vergence equals the horizontal component of the angle formed by the intersection of the two visual axes and it is described with three different units of measurement or scales that include the degree, prism diopter (PD) and meter angle (MA). Prism diopters equal 100 times the tangent of the angle. Meter angles equal the reciprocal of the viewing distance (specified in meters) at which the visual axes intersect as measured from the center of eye rotation. A target at one meter stimulates one meter angle of convergence (approximately 3.4 degrees) and one diopter of accommodation. Prism diopters can be computed from meter angles by the product of MA and the interpupillary distance (IPD), measured in cm. For example, convergence at a viewing distance of 50 cm by two eyes with a 6 cm IPD equals either 2 MA or 12 PD. The advantage to units in MA is that the magnitude of the stimulus to accommodation in diopters is approximately equal to the magnitude of the stimulus to convergence, assuming that both are measured from a common point such as the center of eye rotation. This assumption produces large errors for viewing distances less than 20 cm because accommodation is usually measured in reference to the corneal apex that is 13 mm anterior to the center of eye rotation. The advantage to units in prism diopters is that they are easily computed from the product of MA and IPD. The advantage to units in degrees is that they accurately quantify asymmetric convergence where targets lie at different distances from the two eyes.
The Maddox classification (Maddox, 1893) describes open- and closed-loop components that make up the horizontal vergence response. The classification includes three open-loop components that influence heterophoria. These include an adaptable intrinsic bias (tonic vergence), a horizontal vergence response to monocular depth cues (proximal vergence), and a horizontal vergence response that is coupled with accommodation by a neurological cross-link known as accommodative-convergence. Tonic vergence has an innate divergence posture (5 deg) known as the anatomical position of rest (Robinson, 1975b). Tonic vergence adapts rapidly to compensate for vergence errors during the first 6 weeks of life. At birth the eyes diverge during sleep, but after 6 weeks the visual axes are nearly parallel during sleep (Rethy, 1969). The adapted vergence, measured in an alert state, in the absence of binocular stimulation and accommodative effort, is referred to as the physiological position of rest. The physiological position of rest equals the sum of tonic vergence and the anatomical position of rest. Tonic vergence adaptation ability continues throughout life to compensate for trauma, disease, and optical distortions from spectacles and aging factors.
Proximal vergence describes the open-loop vergence response to distance percepts stimulated by monocular depth cues such as size, overlap, linear perspective, texture gradients and motion parallax (McLin, Schor and Kruger, 1988; Wick and Bedell, 1989). This proximal response accounts for a large portion of the convergence response to changing distance (Judge, 1991; Schor et al 1992). The open-loop convergence response is also increased by efforts of accommodation (Muller 1826; Alpern and Ellen 1956). The eyes maintain approximately 2/3 of a meter angle of convergence (4 prism diopters) for every diopter of accommodation (Ogle et al 1967). A target at one meter stimulates one meter angle of convergence (approximately 3.4 degrees) and one diopter of accommodation. Thus when the eyes accommodate 1 diopter the accommodative-convergence response increases by approximately 2.3 degrees or 4 prism diopters which is only 68 percent of the convergence stimulus. The sum of the three open-loop components of convergence typically lag behind the convergence stimulus and produce an open-loop divergence error (exophoria) that is usually less than two degrees under far viewing conditions and 4 degrees at near viewing distances (Borish, 1970). Esophoria describes open-loop vergence errors caused by excessive convergence that lead the stimulus. The distribution of heterophoria in the general population is not gaussian or normally distributed. It is narrowly peaked about a mean close to zero (Tait, 1933), indicating that binocular errors of eye alignment are minimized by an adaptive calibration process. During closed-loop stimulation, vergence error is reduced to less than 1/10 degree by the component of the Maddox classification controlled by visual-feedback, disparity vergence, which is stimulated by retinal image disparity. Variation in any of the three open-loop components of convergence will influence the magnitude of heterophoria and the resulting closed-loop fixation disparity. Fixation disparity acts as a stimulus to maintain activity of the disparity vergence so that it continues to sustain its nulling response of the underlying heterophoria during attempted steady binocular fixation (Schor, 1983). Other dimensions of vergence also adapt to disparity stimuli. Vertical vergence adapts to vertical prism before one eye (Schor, Gleason, Maxwell, and Lunn 1993) and cyclovergence adapts to optically produced cyclodisparity stimuli (Maxwell and Schor, 1999; Taylor, Roberts and Zee, 2000; Schor Maxwell and Graf, 2001)
B. Dynamic Control of Eye Alignment (Smooth Tracking Responses To Open- and Closed-Loop Stimuli):
1. Conjugate Smooth Pursuit Tracking:
Smooth following pursuit movements allow the eyes to maintain foveal alignment with a moving target that is voluntarily selected (Kowler et al 1984;see Pola and Wyatt, 1991 for a review). Pursuit is defined as the conjugate component of smooth following eye movement responses to target motion. As described below, disconjugate following responses to changes in target distance are referred to as smooth vergence. Conjugate pursuit differs from the delay portion of OKN, which is a reflex response to optic flow of the entire visual field. A conflict occurs between the pursuit and optokinetic systems when the eyes pursue an object that is moving across a stationary background. Pursuit stabilizes the moving target on or near the fovea but it causes optic flow or retinal slip of the stationary background scene. Conflicts can also occur between pursuit and the VOR if gaze is controlled with head tracking movements. Stabilizing the retinal image of a moving object with head movements produces a vestibular signal from the canals. Consequently, pursuit of a small moving target against a stationary background with eye or head movements requires that OKN and the VOR be ignored or suppressed. This is accomplished most effectively when the background lies at a different distance than the pursuit target (Howard and Marton, 1997).
Pursuit responds to target velocities ranging from several minutes of arc/sec to over 175 deg/sec (Pola and Wyatt, 1989). The gain or accuracy of pursuit is reduced as target velocity increases above 100 deg/sec (Myer, Lasker and Robinson, 1985). The VOR has a much higher velocity range than pursuit. This can be demonstrated by comparing two views of your index finger. Either keep your finger stationary while you shake your head rapidly from side to side at 2-3 Hz, or shake your finger at the same frequency without moving your head. The eye cannot follow the moving finger, but it can follow the stationary finger while shaking your head even though the head-relative motion is identical in these two examples. The pursuit response is more accurate when the target motion is predictable such as with pendular motion (Stark et al. 1962). Pursuit errors are reduced by modifying pursuit velocity and with small catch-up saccades. The combination of pursuit and catch-up saccades that appear at low stimulus velocities in patients with pursuit deficits is referred to as cogwheel pursuit. The ratio of eye velocity over target velocity (gain) or accuracy of pursuit is normally affected by target visibility (contrast) as well as drugs and fatigue (O'Mullane and Knox, 1999; Rashbass 1961).
The pursuit response to sudden changes in target velocity has a short latency (80-130 msec) (Lisberger and Westbrook, 1985) and is composed of two general phases referred to as open-loop and closed-loop (Lisberger et al 1985) (Figure 9). Pursuit is initiated during the open-loop phase and it is maintained during the closed-loop phase. The open-loop response is divided into an early and late component. The early component is a feed forward phase that lasts for only 20 msec. During this early phase, there is a rapid acceleration of the eye (40- 100 deg/sec/sec) that is in the correct direction but is independent of the stimulus velocity and initial retinal image position (Lisberger and Westbrook 1985). During the late open-loop component that lasts 80 msec, the initiation of pursuit depends strongly on target velocity and retinal image position (Carl and Gellman, 1987). Eye acceleration is highest in response to targets imaged near the fovea and decreases sharply with increasing eccentricity up to 21 degrees. These open-loop components are calibrated by adaptation (Carl and Gellman, 1986).
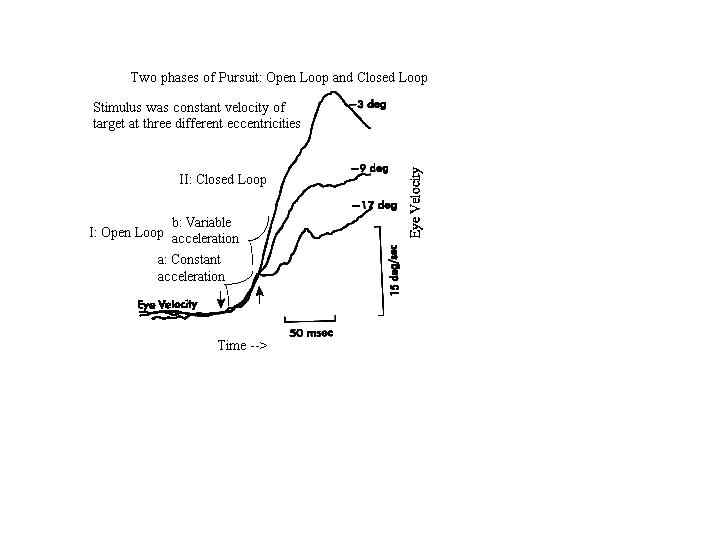
Fig. 9 Eye velocity during the onset of pursuit to a 15 deg/sec ramp target motion, the ramp of motion beginning at different eccentricities as indicated at the right portion of each trace. The velocity of the early component (indicated by arrows) was the same for all starting positions, but the velocity of the late component varied (from Lisberger and Westbrook, (1985)
Pursuit is maintained during the closed-loop phase, in response to negative feedback from retinal image velocity (retinal slip) and position as well as an internal estimate of target velocity relative to the head. If the eye lags behind the stimulus, the retinal image velocity is not nulled leaving a residual retinal image slip and position error away from the fovea. The pursuit system accelerates to correct both retinal position and velocity errors (Rashbass 1961). When pursuit is very accurate and there is no retinal error, the eye continues to pursue the target without the eye-referenced signals. Pursuit is maintained by an internal estimate of target velocity or a head referenced motion signal that is computed from a combination of retinal slip and an internal representation of eye velocity (Pola and Wyatt, 1989). This can be demonstrated by attempting to fixate an ocular floater or a retinal afterimage that is located near the fovea. Attempts to foveate the stabilized retinal image lead to smooth following eye movements even though the retinal image is always motionless. The eye is tracking an internal correlate of its own motion that causes the target to appear to move with respect to the head.
2. Disconjugate Smooth Vergence Tracking:
Foveal alignment of targets that move slowly in depth is maintained by smooth vergence following eye movements (for reviews see Judge, 1991; Collewijn and Erkelens 1990). In addition to improving stereoscopic depth perception, smooth vergence provides information about changing target distance that affects size and depth perception (Hollins and Bunn, 1977; Foley 1978; Bradshaw, Glennerster and Rogers, 1996). Slow changes in smooth vergence respond to body sway and posture instability. While smooth vergence responses can be very inaccurate during natural rapid head movements, when the head is stationary they are very accurate at temporal frequencies up to 1 Hz (Rashbass and Westheimer, 1961). At higher frequencies accuracy is reduced but it can improve with small disparity stimuli (Collewijn and Erkelens, 1990). It is likely that accuracy of smooth vergence is task dependent. Smooth vergence accuracy is more demanding for spatial localization tasks that lack depth cues other than disparity, compared to tasks that have ample monocular cues for direction and distance. The accuracy of smooth vergence responses to changing disparity improves with predictable target motion (Rashbass and Westheimer, 1961). Stimuli for smooth vergence tracking include magnitude and velocity of retinal image disparity (Rashbass and Westheimer, 1961) and perceptual cues to motion in depth, including size looming (Collewijn and Erkelens 1990, McLin et. al, 1988). Smooth vergence tracking is very susceptible to fatigue and central nervous system suppressants (Rashbass and Westheimer, 1961).
3. Adaptable Interactions between Smooth Pursuit and Smooth Vergence:
In natural scenes, motion of a target in the fronto-parallel plane is tracked binocularly with conjugate smooth pursuit. However in conditions of anisometropia corrected with spectacle lenses, the image motion of the two eyes is magnified unequally and this produces variations of binocular disparity that increase with target eccentricity from the optical centers of the lenses. Tracking motion of targets in the fronto-parallel plane then requires both smooth pursuit and smooth vergence eye movements such that one eye moves more than the other does. The oculomotor system can adapt to the binocular disparity that changes predictably with eye position. Adaptation produces open-loop non-conjugate variations of heterophoria that compensate for the horizontal and vertical disparities produced by the unequal magnifiers during smooth vergence tracking responses (Schor Gleason and Horner 1990). After only one hour of pursuit tracking experience with anisometropic spectacles, one eye can be occluded and the two eyes continue to move unequally during monocular tracking (Schor et al 1990). The adapted heterophoria is coupled to vary with both eye position and direction of eye movement (Gleason Schor Lunn and Maxwell 1993).
C. Neuro-Control of Smooth Foveal Tracking
1. Smooth Pursuit Tracking System
Smooth following eye movements result from cortical motion signals in extrastriate cortex in areas MT and MST that lie in the superior temporal sulcus (for reviews see Keller, 1991; Albright, 1989). Area MT encodes speed and direction of visual stimuli in three dimensions relative to the eye. MT receives inputs from the primary visual cortex and projects visual inputs to area MST and the frontal eye fields (FEF). Cells in MST fire in concert with head-centric target movement; i.e. they combine retinal and efference copy signals (Newsome, Wurtz and Komatsu, 1988). Each hemisphere of the MST codes motion to the ipsilateral side. Cells have two types of visual motion sensitivity; they respond to motion of large-field patterns and small spots but the direction preferences for the two stimulus types are in opposite directions. The anti-directional large-field responses could facilitate pursuit of small targets moving against a far stationary field, and motion parallax stimuli. Efference from MST and FEF projects ipsilaterally to the NOT to generate OKN and to the dorsal lateral pontine premotor nuclei (DLPN) for pursuit tracking (Fuchs and Mustari 1993). Velocity signals are projected from DLPN to the floccular region and to the vermis lobules VI and VII of the cerebellum (Fuchs and Mustari 1993). The flocculus is thought to maintain pursuit eye movements during steady constant tracking while the vermis is important when the target velocity changes or when initiating pursuit. The role of the cerebellum is to sort out eye and head rotations in the tracking process and to sort out the ocular pursuit signal from visual and eye-head motor inputs (Noda and Warabi 1987). From here, activity passes via parts of the vestibular nuclei, which perform the necessary neural integration of the velocity signal to a position signal that is sent to the eye muscle motor neurons.
2. Smooth Vergence Tracking System.Vergence results from the combined activity of intrinsic tonic activity, accommodative vergence, and responses to binocular disparity and perceived distance (see Leigh and Zee, 1999 for a review). The sensory afferent signals for vergence (binocular disparity and blur) are coded in the primary visual cortex (area V1) (Poggio, 1995; Busettini ; Miles, 1997). Some cells in V1 incorporate vergence to code egocentric (head-referenced) distance (Trotter, Clelebrini, Stricanne, Thorpe, and Imbert 1996). Cells in area MT and MST respond to retinal disparity and changing size (DeAngelis and Newsome.1999; Maunsell and Van Essen 1983; Roy, Komatsu and Wurtz, 1992). Cells in the parietal cortex respond to motion in depth (Colby, Duhamel and Goldberg, 1993). Efferent commands for vergence appear in cells in the frontal eye fields (Gamlin and Yoon 2000). In the midbrain the premotor nucleus reticularis tegmenti pontis (NRTP), located just ventral to the rostral portion of the paramedian pontine reticular formation (PPRF), receives projections from the frontal eye fields and the superior colliculus (Gamlin and Clark 1995). The NRTP projects to the cerebellum, and appears to be associated with vergence and accommodation (Ohtsuka, Maekawa and Sawa, 1993). The posterior interposed nucleus (PIN) of the cerebellum projects to supraoculomotor regions that contain near response cells in the mesencephalic reticular formation (MRF) (May, Porter and Gamlin 1992; Zhang and Gamlin 1998). The supraoculomotor nucleus contains both burst and tonic neurons (Mays and Porter, 1984). The burst cells code velocity signals for smooth vergence, and the tonic neurons code position signals to maintain static vergence angle. Excitatory connections of the supraoculomotor nucleus project to the oculomotor nucleus, driving the medial recti (Judge and Cumming, 1986; Zang, Mays and Gamlin, 1992). Inhibitory connections project to the abducens nucleus to inhibit the lateral rectus (Mays and Porter 1984). The supraoculomotor nucleus relays control of both accommodative and disparity vergence (Judge and Cumming, 1986).
III.FOVEAL GAZE SHIFTS: TARGET SELECTION AND FOVEAL ACQUISITION.
A. Rapid Conjugate Shifts of Gaze Direction (Saccadic Eye Movements)
Saccades are very fast, yoked eye movements that have a variety of functions (for reviews see Becker 1989 and Van Gisbergen and Van Opstal, 1989). They produce the quick phase of the VOR and OKN to avoid turning the eyes to their mechanical limits. They reflexively shift gaze in response to novel stimuli that appear unexpectedly away from the point of fixation. Saccades shift gaze during reading from one group of words to another. Saccades search novel scenes to assist us in acquiring information. They also return gaze to remembered spatial locations. Two primary functions in all of these tasks are to move the eye rapidly from one position to another and then maintain the new eye position. The rapid movement is controlled by a pulse and slide innervation pattern and the position is maintained by a step innervation.
The separate components of innervation for the saccade match the characteristics of the plant (i.e. the globe, muscles, fat and suspensory tissues). The rapid changes in orbital position are made by the saccade at a cost of considerable energy. Saccade velocities can approach 1000 deg/sec (Becker 1989). In order to achieve these high velocities a phasic level of torque is needed to overcome the viscosity of orbital tissues, most of which is in the muscles (Robinson, 1975). The phasic-torque is generated by a large brief force resulting from a pulse or burst of innervation. The torque is dissipated or absorbed by the muscles, so that the force developed by the pulse of innervation does not reach the tendon (see chapter on Three-Dimensional Rotations of the Eye). At the end of the saccade, a lower constant force resulting from a step innervation generates a tonic level of torque that is needed to hold the eye still against the elastic restoring forces of the orbital tissues (van Gisbergen and Van Opstal, 1989). The eye positions resulting from the pulse and step forces must be equal to produce rapid gaze shifts. Pulse-step mismatches will result in rapid and slow components of gaze shifts. For example, if the pulse is too small, the saccade will slide (post-saccadic drift, called a glissade) to the new eye position at the end of the rapid phase of the saccade. The slide component is adaptable as has been shown by long-term exposure to artificially imposed retinal image slip immediately after each saccade (Optican and Miles, 1985). The adapted post-saccadic drift cannot be explained by an adjustment of the pulse-step ratio, suggesting that the slide innervation is an independent third component of saccadic control (pulse-slide-step). Slide innervation produces a phasic-torque in addition to the pulse component that adjusts the duration and velocity of the saccade so that its amplitude matches the position maintained by the step component (Optican and Miles, 1985). The pulse, slide and step are all under independent, cerebellar control, with the primary goal of protecting vision by preventing retinal slip, and a secondary goal of making accurate saccades.
The amplitude of the saccade determines its dynamic properties (e.g., its peak velocity and duration). The main sequence diagram plots these two dynamic parameters as a function of amplitude (Bahill, Clark and Stark 1975) (Figure 10). As saccade amplitude increases from 0.1 to 10 degrees, duration increases from 20 to 40 msec and peak velocity increases from 10 to 400 deg/sec. Peak velocity saturates for saccades larger than 20 degrees, such that the amplitude of larger saccades increases primarily with duration. Abnormal saccade amplitudes (dysmetria) can either be too small (hypometric) or too large (hypermetric). Large gaze shifts are normally accomplished with a sequence of hypometric saccades that are composed of a series of short-latency corrective saccades in the same direction (Becker 1989). The normal latency of a saccade to an unpredictable stimulus is 180-200 msec (Carpenter, 1988). However, corrective saccades occur with shorter latencies (100-150 msec). Saccade latency can be reduced by a blank or gap interval before the saccade, resulting in an express saccade with latencies less than 100 msec (Fischer and Ramsperger 1984). Saccade latencies to predictable target changes, such as occur in a tennis match, can be reduced to zero (Stark, Vossius and Young, 1962).
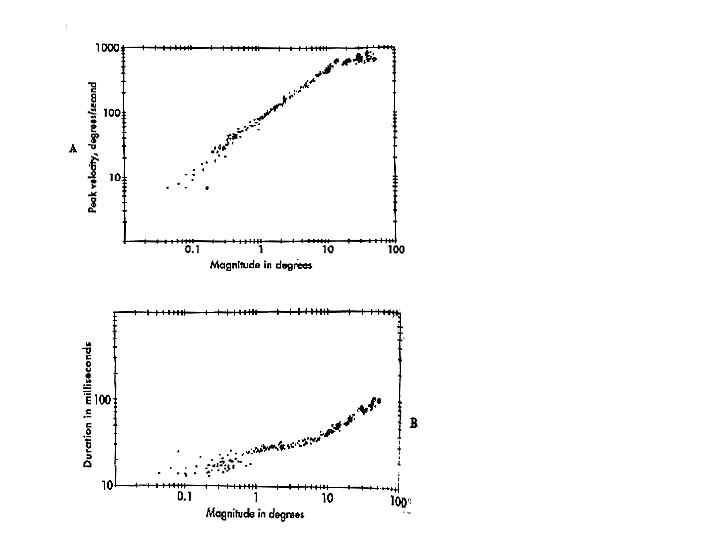
Fig. 10 Main sequence diagram. Peak velocity and saccade duration are plotted against magnitude of human saccadic eye movements. (from Bahill AT, Clark MR and Stark, L: Math Biosci 24:191, copyright 1975 by Elsevier Science Publishing Co, Inc.) Adler’s figure 1992 p 158
Although saccades are too brief to utilize visual feedback during their response, they do use fast internal feedback based upon an internal representation of eye position (efference copy signal) that helps control the position of the eye on a moment-to-moment basis (Robinson, 1975). Thus saccadic eye movements are not ballistic in that they are guided by extra-retinal information during their flight. The goal of the saccade is to reach a specified direction in head-centric space. Normally the perceived head-centric direction of a target does not change when the eye changes position. However spectacle refractive corrections that magnify or minify the retinal image produce changes in perceived direction with eye position. Because the entrance pupil of the eye translates when the eye rotates, prior to the saccade the eye views a non-foveal eccentric target through a different part of the lens than after the saccade when the target is viewed directly along the line of sight. The prismatic power of the lens increases with distance from the optical center of the lens such that when viewing a target through a magnifier, the saccade amplitude needed to fixate an eccentric target is larger than the gaze eccentricity sensed prior to the saccade. Saccades are controlled by a feedforward system that does not utilize visual feedback during the motor response. Consequently, initial saccadic responses to visual distortion produced by the magnifier are hypometric. However, using position errors after the saccade, the system adapts rapidly (within 70 trials) to minimize its errors (Deuble, 1995). In cases of anisometropia in which the retinal images are magnified unequally by the spectacle refractive correction, the saccadic system adapts to produce unequal or disconjugate saccades that align both visual axes with common fixation targets (Erkelens, Collewijn and Steinman, 1989; Oohira, Zee and Guyton 1991; Lemij and Collewijn, 1992). The same adaptive process is likely to calibrate the conjugate saccades and maintain that calibration throughout life in spite of developmental growth factors and injury.
Large abrupt shifts in viewing distance stimulate adjustments of several motor systems including accommodation, convergence and pupil constriction (see Judge, 1989 for a review). Separate control systems initiate and complete these responses (Semmlow, Hung, Horng and Ciuffreda, 1994). Initially the abrupt adjustments are controlled by feedforward systems that do not use visual feedback until the responses are nearly completed. However they are guided by fast feedback from efference copy signals to monitor both the starting point of the near response and the accuracy of its end point (Zee and Levi, 1989; Schor, Alexander, Cormack and Stevenson, 1992). Visual feedback is unavailable during the response because the blur and disparity cues are too large at the beginning of the gaze shift to be sensed accurately. The motor responses are initiated by high-level cues for perceived distance and by voluntary shifts of attention. Retinal cues from blur and disparity are only used as feedback to refine the responses once the stimuli are reduced to amplitudes that lie within the range of visual sensitivity, i.e., as the eyes approach alignment at their new destination. The three motor systems are synchronized or coordinated with one another by cross-links. When they approach their new target destination, accommodation and convergence use visual feedback to refine their response.
The cross-couplings are demonstrated by opening the feedback loop for one motor system while stimulating a coupled motor system that is under closed-loop control. For example, accommodative convergence, measured during monocular occlusion, increases linearly with changes in accommodation stimulated by blur (Muller, 1826 Alpern and Ellen 1956). Similarly, convergence accommodation, measured during binocular viewing through pinhole pupils, increases linearly with changes in convergence stimulated by disparity (Fincham and Walton, 1957). The pupil constricts with changes in either accommodation or convergence to improve the clarity of near objects (Wilhelm; Schaeffel; Wilhelm 1993). These interactions are greater during the dynamic changes in the near response than during the static endpoint of the response (Schor and Kotulak, 1986).
The cross-couplings between accommodation and convergence are illustrated with a heuristic model (Figure 11) (Schor and Kotulak 1986). Three of the Maddox components are represented in the model by an adaptable slow tonic component, the cross-links between accommodation and convergence, disparity driven vergence and blur driven accommodation (fast phasic components). The enhanced gain of the cross-link interactions associated with dynamic stimulation of vergence and accommodation results from the stimulation of the cross-links by the phasic but not the tonic components. When accommodation or convergence is stimulated, the faster transient-phasic system responds first, but it does not sustain its response. The slower and more sustained adaptable tonic system gradually takes over the load of keeping the eyes aligned and focused by resetting the level of tonic activity. Because the cross-links are mainly stimulated by the phasic component, accommodative vergence and vergence accommodation are stimulated more during the dynamic response than during steady fixation when the tonic components control eye alignment and focus.
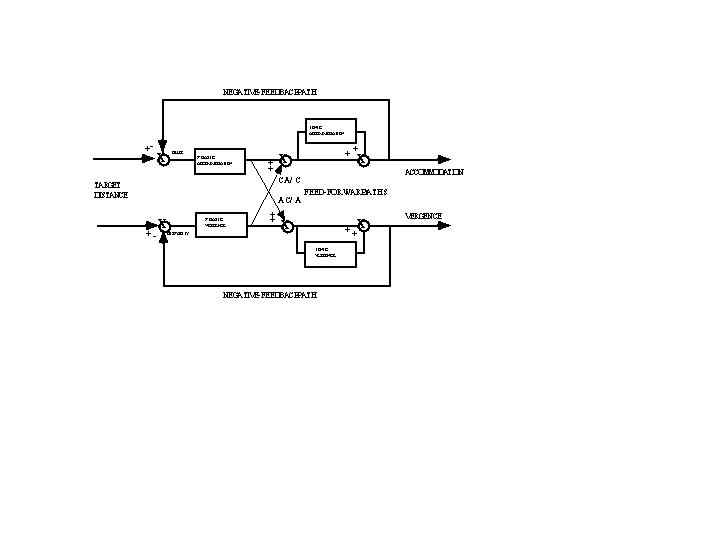
Fig. 11 Model of cross-link interactions between vergence and accommodation. A fast phasic system drives the cross-links from accommodation to convergence (AC/A) and from convergence to accommodation (CA/C). The slow-tonic system adapts to the faster phasic system and gradually replaces it. Cross-link innervation is reduced when the tonic system reduces the load on the fast phasic system. (From Kotulak and Schor, 1986).
Traditionally, only the horizontal component of vergence has been considered as part of the near response. However, vertical and cyclovergence must also be adjusted during the near response to optimize the sensory stimulus for binocular vision (Allen and Carter, 1967; Tweed, 1997; Schor, Maxwell and Stevenson, 1994; Van Rijn and Van den Berg 1993; Yegge and Zee, 1995). The primary goal of the near response is to minimize large changes in horizontal, vertical and cyclodisparities at the fovea that normally accompany large shifts in viewing distance. Horizontal disparities arise from targets that are nearer or farther than the convergence distance, assuming that the horopter equals the Vieth-Muller circle. The isovergence circle describes the locus of points that stimulates a constant vergence angle in all directions of gaze (see Ono, 1983 for a review) and it is equivalent to the Veith-Muller circle that was described in the chapter on Binocular Vision (Figure 2). Convergence and divergence stimuli lie closer or farther, respectively, from the isovergence circle. Vertical disparities arise from targets in tertiary gaze directions at finite viewing distances, because these targets lie closer to one eye than the other and their retinal images have unequal size and vertical eccentricity (vertical disparity) (Figure 12). Torsional disparities arise from elevated targets at finite viewing distances, because during convergence, Listing’s law predicts that the horizontal meridians of the two eyes will be extorted in upward gaze and intorted in downward gaze (Tweed, 1997). These torsional eye postures would produce incyclodisparity in upward gaze and excyclodisparity in downward gaze. With large shifts of viewing distance, horizontal, vertical and cyclodispaities can exceed the stimulus operating range for continuous feedback control of disparity vergence. Initially the near response is stimulated by perceived distance and voluntary changes in horizontal vergence respond without disparity feedback in feedforward control. Unlike horizontal vergence, neither vertical vergence nor cyclovergence is normally under voluntary control. They participate in the near response through cross-couplings with motor responses that are under voluntary control. This allows potential vertical and torsional eye alignment errors to be reduced during the near response without feedback from retinal image disparity.
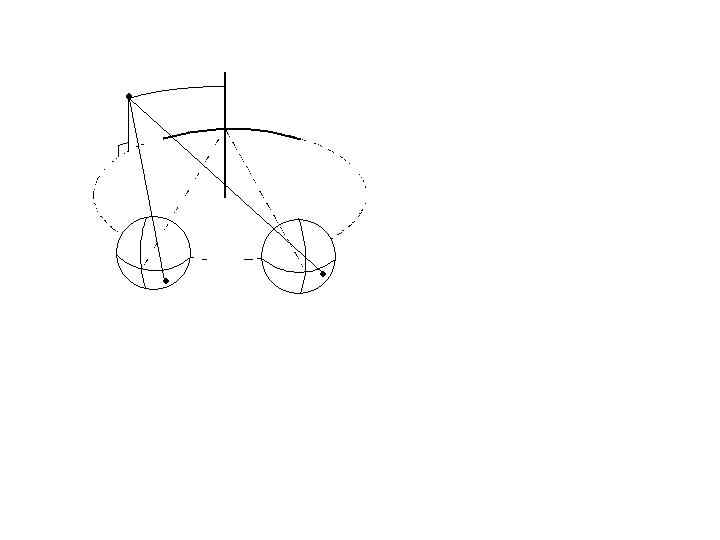
Fig. 12 For convergence at a finite viewing distance, points in tertiary directions subtend unequal vertical visual angles at the two eyes, which produce vertical disparities.
Empirical measures demonstrate that the gains for the three coupling relationships for horizontal, vertical and cyclovergence are optimal for reducing disparity at the fovea to zero during the open-loop phase of the near response (Schor and Kotulak, 1986; Schor, Maxwell Stevenson 1994; Yegge and Zee, 1995; Somani et al., 1998). The tuned coupling gains for all three vergence components of the near response are the product of neural plasticity that adapts each of these cross-couplings to optimize binocular sensory functions (Miles, Judge & Optican 1987; McCandless and Schor, 1997; Schor Maxwell and Graf, 2000). Plasticity also exists for other couplings. For example, vertical vergence and cyclovergence can both be adapted to vary with head roll (Maxwell and Schor, 1997; 1999). The coupling of pupil constriction with accommodation and convergence may also be under adaptive control. The pupil constriction component of the near response does not appear until the end of the second decade of life (Wilhelm, Schaeffel, and Wilhelm 1993) suggesting that it responds to accommodative errors resulting from the aging loss of accommodative amplitude. The pupil constriction component of the near response is an attempt to restore clarity of the near retinal image.
In natural viewing conditions it is rare for the eyes to converge symmetrically from one distance to another. Usually, gaze is shifted between targets located at different directions and distances from the head. Rapid gaze shifts to these targets require a combination of conjugate saccades and disjunctive vergence. In asymmetric convergence, the velocity of disparity vergence, accommodative vergence and accommodation are enhanced when accompanied by gaze shifting saccades (Enright, 1984; Collewijn, Erkelens and Steinman 1997, Schor Lott, Pope Graham 1999). Without the saccade, symmetrical vergence responses are sluggish, reaching velocities of only 10 deg/sec (Rashbass and Westheimer 1961). Symmetrical vergence has a latency of 160 msec and response time of approximately 1 second. Similarly accommodation that is not accompanied by a saccade has a peak velocity of only 4D/sec, a latency of approximately 300-400 msec and response time of approximately 1 second. However when accompanied by a saccade, vergence velocity approaches 50 deg/sec (Collewijn et al, 1997) and accommodation velocity approaches 8-9D/sec (Schor, Lott, Pope and Graham, 1999). Latency of accommodation accompanied by saccades is also reduced by 50 percent so that the accommodative response is triggered in synchrony with the saccade that has a latency of only 200 msec. As shown in Figure 13, response times for both accommodation and accommodative vergence are reduced dramatically when accompanied by saccades. Figure 14 compares symmetric and asymmetric disparity vergence. The high velocity asymmetric vergence appears to result in part from yoked saccades of unequal amplitude. These unequal saccades result from an asynchronous onset of binocular saccades. The abducting saccade begins before the adducting saccade, causing a brief divergence (Maxwell and King, 1992; Zee, FitzGibbon and Optican, 1992). There also appears to be additional accelerated vergence and accommodation responses that are triggered with the saccade (Mays and Gamlin; 1995a; 1995b; Zee et al 1992). Both the vergence and accommodation responses continue after the completion of the saccade but overall the responses are completed in less time than when not accompanied by a saccade.
Fig. 13 Examples of eye movement and accommodation traces during 6 deg rightward Saccade (bottom panels) and No-Saccade (top panels) conditions. (Left panel = trials requiring increased accommodation; Right panel = trials requiring decreased accommodation). Time 0 corresponds to ACStim onset. The following conventions apply: LE = Left (viewing) eye position; RE = Right (non-viewing) eye position; VRG = Vergence position (LE-RE); ACC = Accommodation (D); ACV = Accommodation velocity (D/sec) = derivative of ACC. (Modified from Schor, Lott, Pope and Graham 1999).
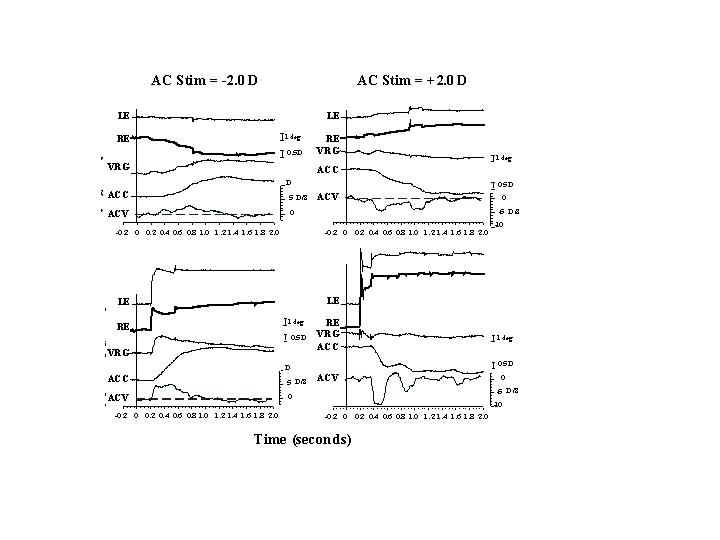
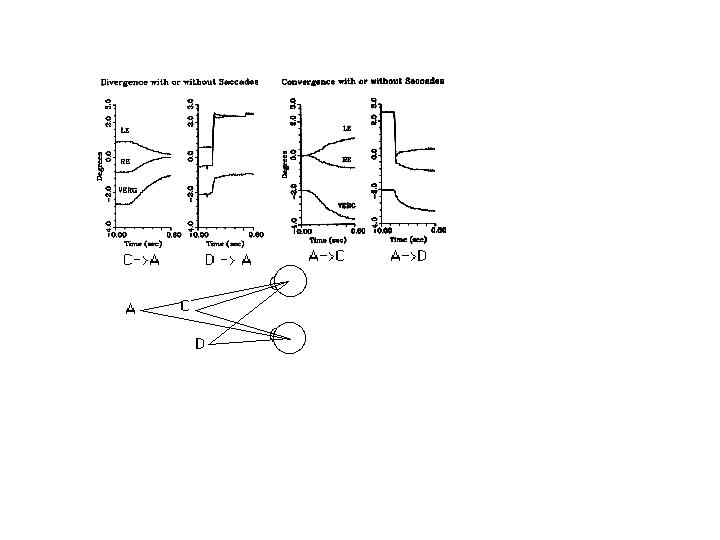
Fig. 14 Vergence changes with or without an accompanying saccade, shown for a rhesus monkey. LE, Left eye; RE, right eye VERG vergence change. Vergence traces (right-left eye position) are offset for clarity. Convergence is negative. Note the increase in vergence velocity when a saccade is conjoined with vergence. The facilitation is greater for divergence because of the inherent divergence associated with horizontal saccades. (From Leigh and Zee 1998)
D. Neuro-Control of Foveal Gaze Shifts:
1. Saccadic Gaze Shifting System
The frontal eye fields (FEF) mediate voluntary control of contralateral saccades. The FEF is active whether saccades occur or not (see Goldberg and Segraves, 1989 for a review). The activity is related to visual attention, and when saccades occur the related activity in the FEF precedes them by 50 msec. The surface of the FEF has a coarse retinotopic organization. Stimulation of a particular area causes a saccade to change eye position in a specific direction and amplitude. These cells are active before saccades to certain regions of visual space. These regions are called the movement field of the cell, and they are analogous to the receptive fields of sensory neurons in the visual cortex. Stimulation of FEF cells in one hemisphere causes conjugate saccades to the contralateral side. Vertical saccades require stimulation of both hemispheres of the FEF. Modalities that can stimulate movement fields include vision, audition, and touch. The FEF project two main efferent pathways for the control of saccades. One projection is to the superior colliculus (SC). The other projection is to the midbrain, to the paramedian reticular formation (PPRF) and the rostral interstitial medial longitudinal fasciculus (riMLF) for the control of horizontal and vertical saccades, respectively (for reviews see Hepp, Henn, Vilis and Cohen 1989; Sparks and Hartwich-Young, 1989). The fibers from the frontal eye fields descend to the ipsilateral superior colliculus and cross the midline to the contralateral PPRF. Neither the superior colliculus nor FEF are required exclusively to generate saccades. Either one of them can be ablated without abolishing saccades, however if both are ablated, saccades are no longer possible. The function of the SC is to represent intended gaze direction resulting from combinations of head and eye position. Stimulation of a specific region in the intermediate layers of the colliculus can result in several combinations of head and eye position that achieve the same gaze direction relative to the body (Freedman and Sparks, 1997). Cells in the superior colliculus respond to all sensory modalities including vision, audition, and touch. The spatial locations of all of these sensory stimuli are mapped in the colliculus relative to the fovea. Like the FEF, stimulation of one SC causes a conjugate saccade to the contralateral side; stimulation of both sides is necessary to evoke purely vertical saccades.
The output of the SC and FEF project to two premotor nuclei, the PPRF and the riMLF, that shape the velocity and amplitude of horizontal and vertical components of saccades, respectively (Hepp et al 1989 Keller, 1991). The PPRF projects to the ipsilateral abducens nucleus, which contains motoneurons that innervate the ipsilateral lateral rectus and interneurons that project to the contralateral oculomotor nucleus to innervate the medial rectus. The PPRF also projects inhibitory connections to the contralateral PPRF and vestibular nucleus to reduce innervation of the antagonist during a saccade. The riMLF projects to the ipsilateral trochlear nucleus (IV) and to both oculomotor nuclei (III). Four types of neurons including burst cells, tonic cells, burst-tonic cells and pause cells control saccades in several premotor sites. The pulse component of the saccade is controlled by medium-lead burst neurons. Long-lead burst neurons discharge up to 200 msec before the saccade and receive input from the SC and FEF. They drive medium-lead burst neurons (MLB) that begin discharging at a high frequency (300-400 spikes/sec) immediately at the beginning of the saccade and throughout its duration. Duration of MLB activity ranges from 10 to 80 msec. They project to the motor nuclei and control pulse duration and firing frequency, which determine saccade duration and velocity. Inhibitory burst neurons inhibit antagonist muscles by suppressing neurons in the contralateral abducens nucleus.
Initiation of the pulse is gated by the omnipause neuron (OPN), which is located in the nucleus of the dorsal raphe, below the abducens nucleus (Figure 15). Normally the OPNs prevent saccades by constantly inhibiting burst cells. The OPN discharges continuously except immediately prior to and during saccades, when they pause. Omnipause neurons engage the saccade by releasing their inhibition of the burst cells. The same OPN inhibit saccades in all directions.
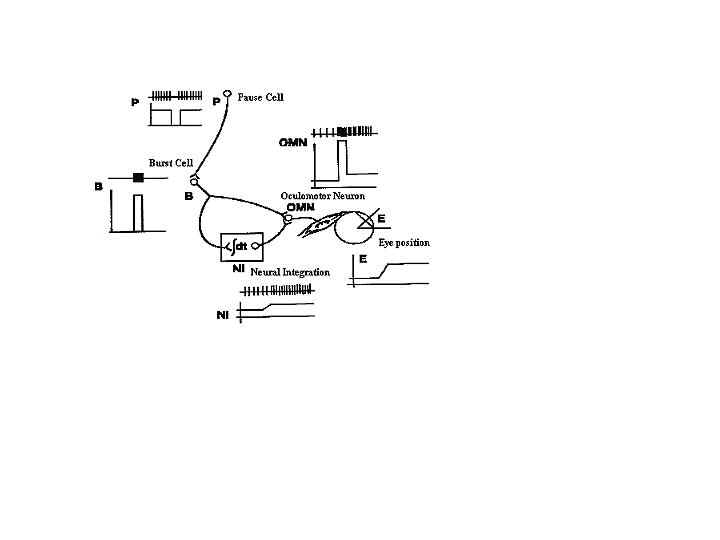
Fig. 15 The relationship among omnipause cells (P), burst cells (B) and cells of the neural integrator (NI), in the generation of the saccadic pulse and step. Omnipause cells cease discharging just before each saccade, allowing the burst cells to generate the pulse. The pulse is integrated by the neural integrator (NI) to produce the step. The pulse and step combine to produce the innervational change on the ocular motoneurons (OMN) that produces the saccadic eye movement (E). Vertical lines represent individual discharges of neurons. Underneath the schematized neural (spike) discharge is a plot of discharge rate versus time.(From Zee and Leigh, 1991)
Upon completion of the saccade, the new eye position is held by the discharge step of the tonic cell. Integrating the pulse derives the discharge rate of the premotor-tonic cell. At least two sites are known to integrate horizontal pulses; these are the medial vestibular nuclei and the nucleus prepositus hypoglossi (NPH). Pulses for vertical saccades are integrated in the interstitial nucleus of Cajal (INC). The flocculus of the cerebellum is also involved in integrating the velocity signals to position signals controlling eye movements. Some anomalies occur that appear to result from lesions of the integrator. In these cases the eyes make a saccade and then drift back to primary position. Affected patients are unable to hold fixation away from primary position and a jerk gaze nystagmus develops in which the slow-phase drift of the eyes is toward primary position and the fast-phase is toward the desired eccentric gaze direction. Combined eye position and velocity signals are carried by burst-tonic neurons. They are active during ipsilateral saccades and inhibited during contralateral saccades.
2. Vergence Gaze Shifting System: The Near Triad and Interactions with Saccades
The supraoculomotor nucleus in the mesencephalic reticular formation contains near response cells. This is a heterogeneous population made up of cells that respond to accommodative stimuli, or vergence stimuli or a combination of accommodation and vergence stimuli (Judge and Cumming, 1986; Zhang et al. 1992; see Mays and Gamlin, 1955a for a review). This nucleus contains burst, tonic and burst-tonic cells that are characteristic of premotor nuclei for saccades. Velocity signals related to disparity stimuli activate burst cells, and a position signal from tonic innervation is derived by integration of the burst cell activity. These cells are believed to provide velocity and position signals to the medial rectus motoneurons in the control of vergence as well as commands to the Edinger-Westphal nucleus to stimulate accommodation (Gamlin et al. 1994). The Edinger-Westphal nucleus, located at the rostral portion of the midbrain at the oculomotor nucleus, contains parasympathetic motor neurons that project to the ciliary muscle, and drive accommodation (Gamlin, et al 1994). Each hemisphere of the Edinger-Westphal nucleus projects to its ipsilateral eye. Parasympathetic outflow of the Edinger-Westphal nucleus also results in miosis (pupillary contraction). Inhibition of the Edinginer-Westphal nucleus causes pupil dilation.
Saccades enhance the velocity of both vergence and accommodation. Current models of saccade-vergence interactions suggest that OPNs in the midbrain gate the activity of both saccade bursters and vergence bursters (Zee et al. 1992). Accelerated vergence caused by saccadic facilitation results from a release from inhibition of vergence bursters by reduced firing of the OPN's, which share their inhibition with saccadic and near response bursters. This facilitation is correlated with an augmentation of firing rate of a sub-set of convergence burst neurons of the near response cells (Mays and Gamlin 1995). This model has been developed further to include the potential for saccadic facilitation of accommodation (Mays and Gamlin (1995 a, b). Because near response cells provide innervation for both accommodation and vergence, release of inhibition from OPNs augments activity of both accommodation and vergence when associated with saccades (Schor, et al 1999). This augmentation would not only enhance velocity of the near response, but it would also gate or synchronize innervation for vergence and accommodation with saccades.
NEUROLOGICAL DISORDERS OF THE OCULOMOTOR SYSTEM
Neurological disorders have greater variability across individuals than is found for the normal oculomotor system. The sources of variability come from the location and extent of lesions, associated anomalies or syndromes, different sources or causes of the problem, history of prior treatment interventions, and adaptive responses that attempt to compensate for the disorder. A multi-perspective classification can reflect this individual variation. It views the anomaly in different ways and highlights very different types of information about the condition. Its versatility describes the range of anomalous behaviors that may develop as a function of various combinations of causal factors. The pattern of associated conditions or syndromes facilitates estimating the prognosis for cosmetic and functional correction. Oculomotor disorders are classified in terms of behavioral descriptions, etiology, and neuro-anatomical sites of involvement. Behavioral categories can be descriptive such as the magnitude and direction of a strabismus (an eye turn), gaze restrictions, saccadic disorders and nystagmus. They can also be described by an associated cluster of anomalies that collectively characterize a disease (syndrome). Categories of etiology include congenital, developmental and acquired. Congenital disorders appear in early infancy (< 18 months). Developmental anomalies can result from interference of normal sensory-motor interactions during the first 6 years of life that constitute the critical period for visual development. Both congenital and developmental disorders are associated with anomalies throughout the oculomotor pathways, including sensory or afferent components of the visual system and they are classified in terms of syndromes. Acquired disorders can result from trauma or disease and they are classified in terms of specific anatomical sites of involvement and by syndromes. (see Leigh and Zee 1999 for a comprehensive review).
I. STRABISMUS:
Non-paralytic strabismus falls into both congenital and developmental categories. A strabismus or tropia describes a misalignment of the two eyes that is not corrected by disparity vergence during binocular fixation. In congenital and developmental forms of strabismus, the eye turn is usually concomitant, meaning that its amplitude does not vary with direction of gaze with either eye fixating. Usually non-paralytic strabismus is a horizontal deviation, where convergent and divergent eye turns are referred to as esotropia and exotropia respectively. Esotropia is more common than exotropia in childhood. Infantile or early-onset esotropia appears within the first 18 months of life and is associated with two forms of nystagmus that include latent or occlusion nystagmus (LN) and asymmetric optokinetic nystagmus, as well as cog-wheel temporalward pursuits (asymmetric pursuit) that were described earlier in this chapter. It is also associated with a vertical misalignment of the eyes when one eye is occluded, that is referred to as dissociated vertical deviation (DVD). When either the right or left eye is occluded, the covered eye rotates upward. This distinguishes DVD from a vertical strabismus in which one of the two eyes remains elevated with respect to the position of the other eye, independent of which eye is fixating. The combination of LN, asymmetric OKN, asymmetric pursuit and DVD are referred to as the infantile strabismus syndrome (Schor et al 1997). The presence of this syndrome in an adult is a retrospective indicator of an early age of onset for a strabismus. The early onset of eye misalignment prevents sensory fusion and causes one eye to be suppressed. This disrupts the development of both monocular and binocular sensory functions and can lead to the development of amblyopia, anomalous correspondence and stereoblindness that are described in the chapter on Binocular Vision. Accommodative esotropia is a developmental form of non-paralytic strabismus that results from either large uncorrected hyperopic refractive errors or abnormally large interactions between the cross coupling between accommodation and convergence. Uncorrected hyperopia produces a mismatch between the stimulus between accommodation and convergence. Excessive convergence is caused by accommodative attempts to clear the retinal image and the cross coupling between accommodation and convergence. The disparity-divergence system is unable to align the eyes and compensate for the accommodative esotropia.
Acquired strabismus usually results from lesions in the brainstem produced by trauma or disease that affect the integrity of the cranial nerves of the final common pathway. The lesions result in muscle palsies that cause deviations between the two visual axes that increase as gaze is directed into the field of action of the affected muscle (paralytic strabismus). The deviation of the paretic eye when the normal eye fixates (primary deviation) is usually smaller than the deviation of the normal eye when the paretic eye fixates (secondary deviation). This non-concomitant variation of eye turn is a diagnostic feature of paralytic strabismus. Lesions of the third, fourth and sixth nerve are referred to as complete ophthalmoplegia, trochlear palsy and abducens palsy, respectively. Ophthalmoplegia describes an immobilized eye resulting from paresis of several muscles innervated by the III nucleus including the medial rectus, vertical recti and inferior oblique as well as the levator of the lid. It is characterized by a fixed-dilated pupil and ptosis, with the eye remaining in a downward and abducted position. Abducens palsy is characterized by an esotropia that increases during abduction of the affected eye. Trochlear palsy is characterized by a hyper deviation of the affected eye that increases during adduction and depression of the affected eye and head tilt to the side of the affected eye.
II. GAZE RESTRICTIONS:
Lesions in premotor nuclei, supranuclear and cortical sites restrict movements of both eyes. The medial longitudinal fasciculus (MLF) is the fiber bundle that interconnects premotor regions with the III, IV and VI cranial nuclei. Any lesion that disconnects these fibers from the premotor to the motor nuclei is referred to as ophthalmoplegia (Figure 16). Lesions caudal to the oculomotor nucleus cause exotropia and failure of adduction, however convergence of the two eyes is spared. Internuclear ophthalmoplegia (INO) refers to an adduction failure caused by disruption of interneuron projections from the abducens nucleus to the contralateral III nerve nucleus. The affected eye is unable to adduct to the contralateral side and the eye drifts in the temporalward direction. Sparing of convergence distinguishes this lesion from complete ophthalmoplegia. Patients with INO can develop a convergence nystagmus in an attempt to bring the exotropic eye into primary gaze. INO is classified as anterior if only adduction of one eye is affected and as posterior if both adduction of one eye and abduction of the other eye are affected. One-and-a-half syndrome is a combination of horizontal gaze palsy and INO that is caused by a lesion of the abducens (affecting the ipsilateral lateral rectus) and interneurons projecting from both abducens nuclei (affecting the ipsilateral and contralateral medial recti).
Fig. 16 Subcortical disorders :gaze palsies. Eye positions shown reflect attempted right gaze in each case, but the arrows show the full range of horizontal gaze for each eye. (Drawing by Scott B Stevenson, courtesy University of Houston).
Foville's syndrome is a unilateral lesion at or near the abducens nucleus which causes conjugate gaze palsy, contralateral limb paralysis, and ipsilateral facial paralysis. Lesions of the abducens nucleus block horizontal movement of both eyes to the side of the lesion because interneurons that project from the abducens nucleus to the contralateral oculomotor nucleus are also affected. Because the abducens is the final common pathway for all lateral conjugate eye movements, lesions there affect saccades, pursuit and the VOR. Lesions in the PPRF only limit horizontal saccades of both eyes to the ipsilateral side and cause drift of the eyes to the contralateral side. Lesions of the DLPN only affect horizontal pursuit toward the side of the lesion.
Lesions rostral to the III nucleus cause paralysis of vertical gaze (Parinaud's syndrome) and failure of convergence, but retention of normal horizontal gaze ability. Parinaud's syndrome occurs with lesions in the vicinity of the riMLF and INC and affects all vertical eye movements including saccades. It often results from tumors of the Pineal gland that compress the superior colliculus and pretectal structures. Unilateral lesions in this area may also cause skew deviations in which there is a vertical deviation of one eye. Unilateral lesions can also cause unilateral nystagmus where the affected eye has a slow upward drift and fast downward saccade (down beat nystagmus).
Cortical lesions produce gaze restrictions of both saccades and pursuits. Lesions of the primary visual cortex produce blindness in the corresponding parts of the visual field contralateral to the lesion (scotoma). Pursuit and saccade targets presented in the scotoma are invisible to the patient. If the entire visual cortex in one hemisphere is destroyed, the vision near the fovea may be intact (macular sparing), allowing the patient to track targets over the full range of eye movements. Like the primary visual cortex, lesions in MT produce contralateral scotomas of the visual field. Saccades to fixed targets may be accurate but pursuit responses to moving targets presented in the affected field will be absent or deficient. Lesions in MST produce visual effects similar to MT, but they also produce a unidirectional pursuit deficit for targets in both visual hemifields moving toward the side of the lesion. Lesions in the posterior parietal cortex (PP) produce attentional deficits, which make pursuit and saccades to small targets more difficult than larger ones. Lesions of the FEF produce a deficit for horizontal pursuit and OKN toward the side of the lesion and saccades to the contralateral side. Lesions to the SEF impair memory-guided saccades.
III. SACCADE DISORDERS
Saccade disorders consist of abnormal metrics (velocity and amplitude), and inappropriate, spontaneous saccades that take the eye away from the target during attempted fixation. Saccades are classified as too fast or slow if their velocity does not fall within the main sequence plot of velocity vs amplitude (Figure 10). However, saccade velocity may be normal while its amplitude is in error. If saccades are too small, the eye could start to make a large saccade and would accelerate to an appropriate high velocity but then be stopped short of the goal by a physical restriction or by rapid fatigue, such as in Myasthenia Gravis. Saccades can also be interrupted by other saccades in the opposite direction (back-to-back saccades) such as observed in voluntary nystagmus. These are truncated saccades. Slow saccades can result from muscle palsies and a variety of anomalies in premotor neurons (see Leigh and Zee, 1999 for a review).
Cerebellar disorders can cause saccade dysmetria (inaccuracy). Saccades can be too large (hypermetric) or too small (hypometric) relative to the target displacement. Inaccuracy can lead to macrosaccadic oscillations when the eye repeatedly attempts to correct its fixation errors with inaccurate saccades. Either the pulse or the step component of the saccade can be inaccurate (Figure 17). If the pulse is too small the saccade will be slow, and if the step is not constant but decays, eye position will drift toward primary position; repeated attempts to fixate eccentrically will result in gaze-evoked nystagmus. If the pulse and step are mismatched, there will be post-saccadic drift or glissade to the final eye position.
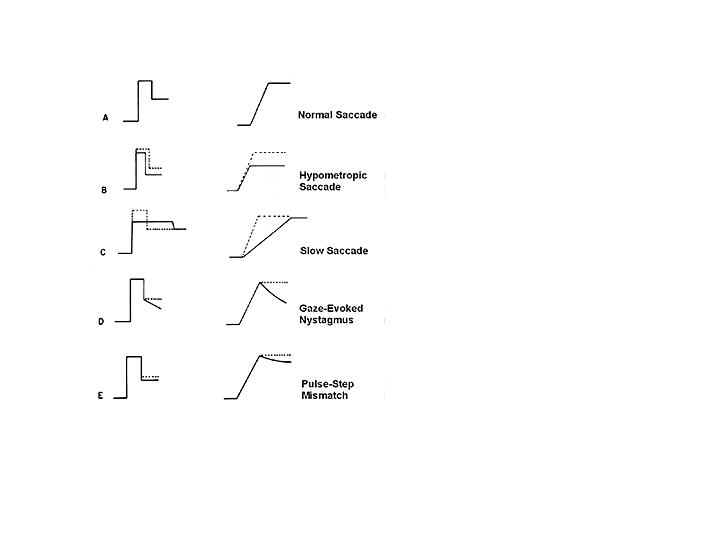
Fig. 17 Disorders of the saccadic pulse and step. Innervation patterns are shown on the left, and eye movements on the right. Dashed lines indicate the normal response. A Normal saccade. B Hypometric saccade: pulse amplitude (width X height) is too small but pulse and step are matched appropriately. C Slow saccade: decreased pulse height with normal pulse amplitude and normal pulse-step match. D Gaze-evoked nystagmus: normal pulse, poorly sustained step. E Pulse-step mismatch (glissade); Step is relatively smaller than pulse. F Pulse-step mismatch due to internuclear ophthalmoplegia (INO): the step is larger than the pulse, and so the eye drifts onward after the initial rapid movements.
Cerebellar disorders and progressive supranuclear palsy can cause saccadic intrusions. These are conditions in which spontaneous saccades occur at the wrong time and move the eye away from the target during attempted fixation. Ocular flutter is characterized by rapid back and forth horizontal saccades without normal saccade latency or intersaccadic interval. Opsoclonus is ocular flutter in all directions. There are also square wave jerks that move the eye away from a point of fixation and then back again. These movements have a normal intersaccadic interval.
IV. NYSTAGMUS.
Nystagmus refers to a regular pattern of to and fro movements of the eyes, usually with alternating slow and fast phases (Figure 6). The direction of the nystagmus is specified according to the direction of the fast phase (e.g. "jerk right"), because the fast phase is more visible than the slow phase. When the velocity of the eye oscillations is equal in both directions the waveform is classified as pendular.
Congenital nystagmus appears early in life and is often associated with albinism, aniridia and congenital acromatopsia. It is a jerk waveform of nystagmus that has a null point or gaze direction where the amplitude is minimized. The differentiating characteristic of CN is that the speed of the slow phase increases exponentially, until a resetting quick phase occurs. In all other forms of nystagmus, the speed of the slow phase is constant or decreases before the quick phase. Persons with CN can adopt a head turn to allow their gaze direction to coincide with the null point. Convergence also dampens CN and in some cases individuals adopt an esotropia in an attempt to block the nystagmus at the expense of binocularity (nystagmus blocking syndrome). Latent nystagmus described earlier in this chapter is a developmental form of nystagmus associated with early-onset esotropia or abnormal binocular vision and it is thought to be related to asymmetric OKN that is also associated with disrupted development of binocular vision (Schor, 1993).
Vestibular nystagmus can result from central and peripheral anomalies of the vestibular gaze stabilization system. The vestibular system is organized in a push-pull fashion where the inputs from each side of the head are normally balanced when the head is stationary. When an imbalance exists, the eyes behave as if the head were constantly rotating. The amplitude of the nystagmus is highest when gaze deviates in the direction of the fast phase (Alexander’s law). In some cases of vestibular nystagmus, the direction of the nystagmus reverses every two minutes (periodic alternating nystagmus, PAN). The reversal reflects the action of the normal cerebellar adaptive control mechanism to correct an imbalance in the vestibular system. Rebound nystagmus is a related condition associated with gaze-evoked nystagmus that was described earlier in this chapter. In gaze-evoked nystagmus, when fixation is held eccentrically, the slow phase is toward primary position. In rebound nystagmus, the amplitude of the nystagmus is reduced after 35 seconds of sustained fixation. However, when the eyes return to primary position, the nystagmus reverses direction (rebound nystagmus). The attenuation and reversal of slow phase direction demonstrates an attempt by the cerebellum to reduce the slow drifts during attempted steady fixation caused by a leaky tonic innervation for eye position.
Nystagmus can also have vertical and torsional components. For example, see-saw nystagmus is an acquired pendular from of nystagmus in which there is a combination of vertical and torsional oscillation of eye orientation. The eyes bob up and down by several degrees in opposite directions (skew movements). As one eye elevates and intorts, the other eye depresses and extorts. The exact cause is unknown, but see-saw nystagmus is associated with visual loss in bitemporal hemianopia and optic chiasm disorders. The nystagmus is thought to result from an inappropriate ocular tilt response (ocular counter-roll during head tilt) orchestrated by the cerebellum in association with the otoliths.
All of the oculomotor anomalies described above show some evidence of attempts by the oculomotor system to correct them using the same adaptive mechanisms that normally calibrate the various classes of eye movement systems. However the anomalies are beyond the corrective range of the adaptive processes. If it were not for these adaptive processes, these anomalies would be far more prevalent and the oculomotor system would be extremely susceptible to permanent injury resulting from disease and trauma. The consequences would be very dramatic. For example, about 50 years ago a physician named John Crawford lost function of his vestibular apparatus as a result of an overdose of streptomycin used to treat tuberculosis located in his knee. He reported that every movement of his head caused vertigo and nausea, even when his eyes were open. If his eyes were shut, the symptoms intensified. He attempted to steady his head by lying on his back and gripping the bars at the head of the bed. However even in this position the pulse beat in his head became a perceptible motion, disturbing his equilibrium. It is difficult to imagine how we would survive without the adaptive processes that continually calibrate our oculomotor system and allow us to distinguish between motion of our head and eyes from motion of objects in the world.
Acknowledgements: Comments by James Maxwell and Lance Optican are appreciated.
REFERENCES
Able LA, Parker L, Daroff RB, Dell’Osso LF: Endpoint nystagmus. Invest Ophthalmol Vis Sci 1977; 17: 539-544
Albright TD : Cortical processing of visual motion. Chapter 9 In.. Miles FA, Wallman J. (eds.): Visual Motion and its role in the Stabilization of Gaze p 177-201 Elsevier Science Publishers B.V.,1993
Allen, MJ & Carter JH :The torsion component of the near reflex. Am. J. Optom1967; 44: 343-349
Alpern M, Ellen P : A quantitative analysis of the horizontal movements of the eyes in the experiments of Johannes Muller. I. Methods and results. Am J Ophthalmol 1956; 42; 289-303
Badcock DR, Schor CM: Depth-increment detection function for individual spatial channels. J Opt Soc Am, 2A, 1985; 1211-1215
Bahill AT, Clark MR, Stark L : The main sequence, a tool for studying human eye movements. Mathematical Biosciences 1975; 24: 191-204
Bahill AT; Adler D; Stark L.: Most naturally occurring human saccades have magnitudes of 15 degrees or less. Investigative Ophthalmology, 1975 14(6):468-9.
Baloh RW, Sharma S, Moskowitz H, Griffith R : Effect of alcohol and marijuana on eye movements. Aviat. Space Environ Med. 1979; 50; 18-23
Becker W : Chapter 2 In Wurtz RH, Goldberg ME (Eds.) The neurobiology of saccadic eye movements. p13-68 Elsevier Science Publishers BV, Amsterdam 1989
Borish IM : Clinical refraction. 3rd ed. p 910 Chicago: Professional Press: 1970
Bradshaw MF, Glennerster A, Rogers BJ : The effect of display size on disparity scaling from differential perspective and vergence cues. Vision Research1996; 36: 1255-1264
Carl JR, Gellman RS: Adaptive Responses in human smooth pursuit. In Keller EL and Zee DS (eds.): "Adaptive processes in visual and oculomotor systems"., Advances in the biosciences Volume 57. p 335-339 Pergamon PressOxford. 1986
Cheng M, Outerbridge JS: Optokinetic nystagmus during selective retinal stimulation. Exp. Brain Res. 1975; 23: 129-139
Carpenter RHS : Movements of the eyes, 2nd ed. London: Pion 1988
Cheung BSK, Howard, IP :Optokinetic torsion: Dynamics and relation to circular vection. Vision Research1991; 31, 1327-1335
Cogan DG: Neurology of the Ocular Muscles, Ed 2. Charles C Thomas, Springfield, Illinois 1956
Colby CL Duhamel JR, Goldberg ME : Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol1993; 69: 902-914
Collewijn H : Integration of adaptive changes of the optokinetic reflex, pursuit and the vestibulo-ocular reflex. Chapter 3 In Berthoz A, Melville Jones G (eds.) Adaptive Mechanisms in Gaze Control. p51-70. Elsevier Amsterdam 1985
Collewijn H, SteinmanRM, Erkelens CJ: Binocular fusion, stereopsis and stereoacuity with a moving head. In Regan D, Ed. Binocular Vision. P 121-136. Macmillian London 1991
Collewijn H, Erkelens CJ: Binocular eye movements and the perception of depth. Chapter 4 in Kowler E (ed.) "Eye Movements and their Role in Visual and Cognitive Processes" p 213-261. Elsevier Science Publishers BV 1990
Collewijn H; Erkelens CJ; Steinman RM.: Trajectories of the human binocular fixation point during conjugate and non-conjugate gaze-shifts. Vision Research, 1997; 37(8):1049-69
Collewijn H :The optokinetic contribution. Chapter 3 In Carpenter RHS (Ed). "Vision and visual dysfunction, vol 8, Eye movements". p 45-70 Boca Raton, Boston, CRC press 1989
DeAngelis GC; Newsome WT.: Organization of disparity-selective neurons in macaque area MT. Journal of Neuroscience, 1999; 19(4):1398-415.
Dell’Osso,LF Schmidt D, Daroff RB : Latent, manifest latent and congenital nystagmus. Arch Ophthalmol1979; 97: 1877-1885
Deuble H: Separate adaptive mechanism for the control of reactive and volitional saccadic eye movements. Vision Res. 1995; 35; 3520-3540
Erkelens, CJ, Collewijn H, Steinman RM : Asymmetrical adaptation of human saccades to anisometropia spectacles. Invest Ophthalmol. Vis. Sci., 1989; 30; 1132-1145
Enright JT: Changes in vergence mediated by saccades. J Physiol. (Lond) 1984;350:9-31
Fincham EF, Walton J: The reciprocal actions of accommodation and convergence. J. physiol. Lond. 1957;137; 488-508
Fischer B, Ramsperger E: Human express saccades: extremely short reaction times of goal directed eye movements. Exp. Brain Res. 1984; 57; 191-195
Foley JM : Primary distance perception. Chapter 6 In Held R Leibowitz HW, Teuber H-L (eds.)Handbook of Sensory Physiology Vol VIII Perception. p181-214. Springer Verlag, Berlin 1978
Freedman EG; Sparks DL.: Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. Journal of Neurophysiology, 1997; 78(3):1669-90.
Fuchs AF, Mustari MJ: The optokinetic response in primates and its possible neuronal substrate. Chapter 16 in. Miles FA, Wallman J (eds )Visual Motion and its role in the Stabilization of Gaze. p 343-369. Elsevier Science Publishers B.V. 1993
Gamlin PD Zhang H Clendaniel RA, Mays LE: Behavior of identified Edinger-Westphal neurons during ocular accommodation. J Neurophysiol 1994;72: 2368-2382
Gamlin PD; Clarke RJ.: Single-unit activity in the primate nucleus reticularis tegmenti pontis related to vergence and ocular accommodation. Journal of Neurophysiology, 1995; 73(5):2115-9.
Gamlin PD, Yoon, K : An area for vergence eye movement in primate frontal cortex. Nature 2000; 407: 1003-1007.
Gleason, G, Schor CM Lunn R, Maxwell, J.: Directionally selective short-term non-conjugate adaptation of vertical pursuits. Vision Research. 1993; 33(1), 65-71
Goldberg ME, Segraves MA : The visual and frontal cortices. Chapter 7 in . Wurtz RH, Goldberg ME.( Eds) The Neurobiology of Saccadic Eye Movements. p 283-313 Elsevier Amsterdam1989
Goldberg ME, Eggers HM, Gouras P : The ocular Motor System. Chapter 43 in Kandel ER, Schwartz JH, Jessell TM (eds.) Principles of Neural Science. Third Edition. p 660-677 Appleton & Lange Norwalk Connecticut. 1991
Hepp, K Henn, V Vilis T, Cohen B: Brainstem regions related to saccade generation. Chapter 4 in Wurtz RH, Goldberg ME (Eds ): The Neurobiology of Saccadic Eye Movements p 105-212. Elsevier Amsterdam. 1989
Hoffmann KP, Distler C, Ilg U: Collosal and superior temporal sulcus contributions to receptive field properties in the macaque monkey’s nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract. J. Comp. Neurol. 1992, 321: 150-162
Hollins M; Bunn KW.: The relation between convergence micropsia and retinal eccentricity. Vision Research, 1997; 17(3):403-8.
Howard IP, Marton, C: Visual pursuit over textured backgrounds in different depth planes. Exp Brain Res 1997; 90: 625-629
Hering E : The theory of binocular vision. 1868 In Bridgeman B, Stark L,(eds.) Plenum Press New York 1977
Ito M Synaptic plasticity in the cerebellar cortex that may underlie the vestibulo-ocular adaptation. Chapter 14 in Berthoz A, Melville Jones G (eds.) Adaptive Mechanisms in Gaze Control. p 213-221 Elsevier Amsterdam 1985
Judge SJ: Vergence. Chapter 7 In Carpenter RHS (ed.): "Vision and visual dysfunction, vol 8, Eye movements". p 157-170 Boca Raton, Boston, CRC press 1991
Judge S, Cumming, B : Neurons in the monkey midbrain with activity related to vergence eye movements and accommodation. J Neurophysiol. 1986; 55: 915-930
Keller, EL : Gain of the vestibulo-ocular reflex in monkey at high rotational frequencies. Vision Research, 1978 ;20: 535-538
Keller EL : The brainstem. In Carpenter RHS (ed.): "Vision and visual dysfunction, vol 8, Eye movements" 200-223 Boca Raton, Boston, CRC press 1991
Kotulak, J., Schor, C.M. .: The dissociability of accommodation from vergence in the dark. Invest. Ophthal. and Vis. Sci. 1986; 27:544-551
Kowler E, van der Steen J, Tamminga EP, Collewijn H : Voluntary selection of the target for smooth eye movement in the presence of superimposed, full-field stationary and moving stimuli. Vision Research 1984; 12: 1789-1798
Kowler E : The stability of gaze and its implications for vision. Chapter 4 In Carpenter RHS (ed.):. "Vision and visual dysfunction, vol. 8, Eye movements". p 71-94 Boca Raton, Boston, CRC press 1989
Land MF : Predictable eye-head coordination during driving. Nature 1992; 359: 318-320
Leigh JR, Zee DS : The Neurology of Eye Movements. Third Edition. Contemporary Neurology Series Oxford University Press, Oxford 1999
Lemij HG; Collewijn H. : Nonconjugate adaptation of human saccades to anisometropic spectacles: meridian-specificity. Vision Research, 1992; 32(3): 453-64.
Lisberger, SG: The latency of pathways containing the site of motor learning in the monkey vestibulo-ocular reflex. Science1984; 225: 74-76
Lisberger SG, Westbrook LE: Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neuroscience, 1985; 5, 1662-1673
Lisberger SG: The neural basis for learning of simple motor skills. Science 1988; 242: 728-735
Maddox EC: The clinical use of prism. 2nd ed. p 83 Bristol England: John Wright & Sons, 1893
Maunsell JHR, Van Essen DC : Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol1983; 49: 1148-1167
Maxwell, J, Schor, CM.: Mechanisms of vertical phoria adaptation revealed by time-course and two-dimensional spatiotopic maps. Vision Research. 1994; 34(2), 241-251
Maxwell JSM, Schor CM : Head-position-dependent adaptation of non concomitant vertical skew. Vision Research 1997; 37:441-446
Maxwell, JS, Schor CM : Adaptation of torsional eye alignment in relation to head roll. Vision Research, 1999, 39(25):4192-4199.
Maxwell, JS, King WM: Dynamics and efficacy of saccade-facilitated vergence eye movements in monkeys. J Neurophysiol. 1992; 68: 1248-1260
May PJ; Porter JD; Gamlin PD.: Interconnections between the primate cerebellum and midbrain near-response regions. Journal of Comparative Neurology, 1992; 315(1):98-116.
Mays LE; Gamlin PD.: Neuronal circuitry controlling the near response. Current Opinion in Neurobiology, 1995a; 5(6):763-8.
Mays LE; Gamlin PD.: A neural mechanism subserving saccade-vergence interactions. In Findlay, Walker, Kentridge (eds.):. Eye movement research: mechanisms, processes and applications. New York: Elsevier1995b
Mays LE, Porter JD : Neural control of vergence eye movements: activity of abducens and oculomotoneurons. J. Neurophysiol.1984; 52: 743-761
McCandless JW, Schor CM : An association matrix model of context-specific vertical vergence adaptation. Network: Comput. Neural Syst. 1997; 8: 239-258
McLin, L., Schor, C.M., Kruger, P.: Changing size (looming) as a stimulus to accommodation and vergence. Vision Res. 1988; 28, 883-898
Melvill Jones G. : The vestibular contribution. Chapter 2 In Carpenter RHS (ed.): "Vision and visual dysfunction, vol 8, Eye movements".p 13-44 Boca Raton, Boston, CRC press 1989
Miles FA: The sensing of rotational and translational optic flow by the primate optokinetic system. In: Miles FA, Wallman J (eds.): Visual Motion and its Role in the Stabilization of Gaze.p 393-403 Amsterdam: Elsevier, 1993
Miles FA, Kawano K ,Optican LM: Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J Neurophysiol. 1986; 56: 1321-1354
Miles FA Judge SJ, Optican LM : Optically induced changes in the couplings between vergence and accommodation. J Neurosci. 1987; 7: 2576-2589
Muller J : Elements of Physiology. Vol 2. Baly W, tras. London: Tylor & Walton. 1826
Myer CH, Lasker AG Robinson DA: The upper limit for smooth pursuit velocity. Vision Research 1985, 25: 561-563
Newsome, WT Wurtz RH, Komatsu, H: Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J. Neurophysiol1988; 60: 604-620
Noda H, Warabi T : Responses of Purkinje cells and mossy fibers in the flocculus of the monkey during sinusoidal movements of a moving pattern. J Physiol. (Lond.) 1987; 387: 611-628
Ogle KN Martens, TG, Dyer JA: Oculomotor imbalance in binocular vision and fixation disparity. Philadelphia: Lea Febiger 1967
Ohtsuka, K Maekawa H, Sawa, W : Convergence paralysis after lesions of the cerebellar peduncles. Ophthalmologica 1993; 206: 143-148
O'Mullane, G; Knox, PC. : Modification of smooth pursuit initiation by target contrast. Vision Research, 1999; V39 N20: 3459-3464.
Ono H () The combination of version and vergence. Chapter 11 in Schor CM, Ciuffreda K (eds.): "Vergence Eye Movements: Basic and Clinical Aspects" p 373-400 Butterworth, Boston 1983
Oohira A; Zee DS; Guyton DL. : Disconjugate adaptation to long-standing, large-amplitude,spectacle-corrected anisometropia. Investigative Ophthalmology and Visual Science, 1991; 32(5):1693-703.
Optican LM, Miles FA : Adaptive properties of the saccadic system. Visually induced adaptive changes in primate saccadic oculomotor control signals. J. Neurophysiol. 54: 940-958
Poggio G: Mechanisms of stereopsis in monkey visual cortex. Cereb. Cortex 1985;3: 195-204
Pola J, Wyatt HJ : Smooth pursuit response characteristics, stimuli and mechanisms. Chapter 6 In Carpenter RHS (ed.): "Vision and visual dysfunction, vol. 8, Eye movements" p 138-156. Boca Raton, Boston, CRC press 1989
Raphan T, Cohen B: Velocity storage and the ocular response to multidimensional vestibular stimuli. Chapter 8 in Berthoz A, Melville Jones G (eds.): Adaptive Mechanisms in Gaze Control. 123-144. Elsevier Amsterdam 1985
Rashbass C, Westheimer G : Disjunctive eye movements. J. Physiol. (Lond.) 1961;159: 339-360
Rashbass C : The relationship between saccadic and smooth tracking eye movements. J. Physiol 1961;159; 326-338
Rethy, I : Development of the simultaneous fixation from the divergent anatomic eye-position of the neonate. J. Ped. Ophthal. 1969; 6 (2), 92-96
Robinson DA, Keller EL : The behavior of eye movement motoneurons in the alert monkey. Bibl. Ophthalmol, 1972; 82: 7-17
Robinson DA : The mechanics of human smooth pursuit eye movements. J. Physiol. 1965; 180: 569-591
Robinson DA : Oculomotor control signals. In: G. Lennerstrand, P. Bach-Y-Rita (eds.): Basic Mechanisms of Ocular Motility and Their Clinical Implications. p 337-374 Pergamon Press, Oxford, 1975a
Robinson DA: Control of eye movements. In: V. Brooks (Ed.) Handbook of Physiology Section I: The Nervous System, The p 1275-1320. The William and Wilkins Co., Bethesda, MD 1981
Robinson, DA: A quantitative analysis of extraocular muscle cooperation and squint. Invest Ophthalmol, 1975b; 14: 801-825
Roy J-P, Wurtz RH: The role of disparity-sensitive cortical neurons in signaling the direction of self-motion. Nature 1990; 348: 160-162
Schor, C.M, Maxwell, J., Stevenson, S.B.: Isovergence surfaces: the conjugacy of vertical eye movements in tertiary positions of gaze. Ophthalmic and Physiological Optics. 1994; 14; 279-286
Schor CM : Fixation disparity and vergence adaptation Chapter14 in Schor CM, Ciuffreda K (eds.): "Vergence Eye Movements: Basic and Clinical Aspects" p 465-516 , Butterworth, Boston, 1983
Schor, CM, Gleason, G, Maxwell, J, Lunn R: Spatial aspects of vertical phoria adaptation. Vision Research. 1993; 33(1), 73-84
Schor, C.M., Gleason, J, Horner D.: Selective Nonconjugate Binocular Adaptation of Vertical Saccades and Pursuits. Vision Res. 1990; 30(11): 1827-1845
Schor, C.M., Kotulak, J.: Dynamic interactions between accommodation and convergence are velocity sensitive. Vision Res., 1986; 26:927-942.
Schor, C.M. Binocular Sensory Disorders. Ed., M. Regan "Vision and Visual Disorders: Vol 9, Binocular Vision pp 179-224. Cronely Dillon, Jr Pub.The Macmillian Press 1991
Schor CM, Lott L, Pope D, Graham A :Saccades reduce latency and increase velocity of ocular accommodation. Vision Research 1999; 39/22; 3769-3795
Schor, CM, Alexander J, Cormack L, Stevenson S : Negative feedback control model of proximal convergence and accommodation. Ophthalmic and Physiological Optics1992; 12: 307-318
Schor CM : Development of OKN. Chapter14 in. Miles FA, Wallman J (eds.):Visual Motion and its role in the Stabilization of Gaze. p 301-320 J. Elsevier Science Publishers B.V. 1993
Schor, C.M, Maxwell, J. & Stevenson, S.B.: Isovergence surfaces: the conjugacy of vertical eye movements in tertiary positions of gaze. Ophthalmic and Physiological Optics. 1994; 14; 279-286
Schor CM, Wilson N, Fusaro,R, McKee S.: Prediction of early onset esotropia from the components of the infantile squint syndrome. Investigative Ophthalmology and Vision Science. 1997; 38:719-740
Schor CM, Maxwell, JS, Graf E: Plasticity of distance-dependent variations of cyclovergence with vertical gaze. Vision Res. 2001 In Press.
Schor CM. Binocular Vision in De Valois K Ed. Seeing. P 177-257. Academic Press 2000
Seidman SH, Leigh RJ: The human torsional vestibulo-ocular reflex during rotation about an earth-vertical axis. Brain Research 1989; 504; 264-268
Semmlow, JL Hung, GK Horng JL, Ciuffreda, KJ : Disparity vergence eye movements exhibit preprogrammed motor control. Vision Research1994; 34: 335-343
Sherrington, C : The integrative action of the nervous system, 2nd ed. New Haven: Yale University Press 1947
Simpson JI, Graf W : The selection of reference frames by nature and its investigators. Chapter 1 in Berthoz A, Melville Jones G (eds.) Adaptive Mechanisms in Gaze Control.p 3-20. Elsevier Amsterdam 1985
Snyder LH, King WM : Effect of viewing distance and the location of the axis of rotation on the monkey’s vestibulo-ocular reflex (VOR). I. Eye movement responses. J Neuro-physiol, 1992; 67; 861-874
Somani RAB, Desouza, JFX Tweed D, Vilis T : Visual test of listing’s law during vergence. Vision Research 1998;38, 911-923
Sparks DL, Hartwich-Young R : The deep layers of the superior colliculus. Chapter 5 in Wurtz RH, Goldberg ME. (eds.): The Neurobiology of Saccadic Eye Movements. p 213-256 Elsevier Amsterdam. 1989
Stark L, Vossius, G Young LR : Predictive control of eye tracking movements. IRE Trans. Hum Factors Electron. 1962; 3: 52-56
Tait EF : A report on the results of the experimental variation of the stimulus conditions in the responses of the accommodative convergence reflex. Am J Optom 1933; 10: 428-435
Taylor MJ; Roberts DC; Zee DS.: Effect of sustained cyclovergence on eye alignment: rapid torsional phoria adaptation. Investigative Ophthalmology and Visual Science, 2000; 41(5):1076-83.
Trotter, Y Clelebrini S Stricanne B Thorpe S , Imbert M: Neural processing of stereopsis as a function of viewing distance in primate visual cortical area V1. J Neurophysiol.1996; 76; 2872-2885
Tweed D : Visual-motor optimization in binocular control. Vision Research 1997; 37, 1939-1951
Van Rijn LJ; Van der Steen J; Collewijn H.: Instability of ocular torsion during fixation: cyclovergence is more stable than cycloversion. Vision Research, 1994 ; 34(8):1077-87.
Van Gisbergen JAM, Van Opstal AJ. :Models, Chapter 3 In Wurtz RH, Goldberg M (eds.): The neurobiology of saccid eye movements. p 69-101 Elsevier Science Publishers BV, Amsterdam 1989
Wallman J : Subcortical optokinetic mechanisms. Chapter 15 In. Miles FA, Wallman J. (eds ):Visual Motion and its role in the Stabilization of Gaze p 321-342 Elsevier Science Publishers B.V. 1993
Warwick R : Representation of the extra-ocular muscles in the oculomotor nuclei of the monkey: J Comp Neurol1953; 98: 449
Westheimer G, McKee SP : Visual acuity in the presence of retinal image motion. J. Opt. Soc Am. 1975; 65: 47-850
Westheimer G, Blair S: The parasympathetic pathways to internal eye muscles. Invest Ophthalmol 1973;12: 193-197
Wick B; Bedell HE.: Magnitude and velocity of proximal vergence. Investigative Ophthalmology and Visual Science, 1989 ; 30(4):755-60.
Wilhelm H; Schaeffel F; Wilhelm B.: Age relation of the pupillary near reflex. Klinische Monatsblatter Fur Augenheilkunde, 1993 ; v203 n2:110-116.
Ygge J; Zee DS. : Control of vertical eye alignment in three-dimensional space. Vision Research, 1995; 35(22):3169-81.
Zee DS; FitzGibbon EJ; Optican LM. : Saccade-vergence interactions in humans. Journal of Neurophysiology, 1992; 68(5):1624-41.
Zee DS, Levi L: Neurological aspects of vergence eye movements. Rev. Neurol. (Paris) 1989;145: 613
Zhang Y; Mays LE; Gamlin PD. : Characteristics of near response cells projecting to the oculomotor nucleus. Journal of Neurophysiology, 1992 ; 67(4):944-60.
Zhang H; Gamlin PD.: Neurons in the posterior interposed nucleus of the cerebellum related to vergence and accommodation. I. Steady-state characteristics. Journal of Neurophysiology, 1998 ; 79(3):1255-69.